1. Introduction
Anatase-TiO2 with wide energy band gap about 3.2 eV has been candidate of transparent conductive oxide (TCO) layer instead of indium-tin-oxide (ITO) as demonstrated by laser-ablation and reactive-sputtering [1] [2] . In the results of such physical vapor deposited layer, it was reported that Nb is a sufficient donor-dopant but resistivity of the doped layer is drastically reduced to 1 × 10−3 Ω∙cm or lower after the post-annealing in reduction-ambient including in vacuum. Therefore, it is considered that oxygen-vacancy (VO) induced by the post- treatment contributes to reducing the resistivity as shown by XPS results [3] , in which the VO probably generates complex center with Nb-donor or Ti3+ which forms Ti-3d1 band [4] . On the other, we demonstrated low-pressure chemical vapor deposition (LPCVD) of anatase-TiO2 layer by using titanium-tetra-iso-propoxide (TTIP: Ti(O-i-C3H7)4) as the metalorganic precursor and NbF5 to reduce the resistivity in O2- and H2-ambient [5] -[7] . It was found in the results of Nb and F co-doped layer that F substituted to the O-site (FO) contributes to the reduction of resistivity without VO [5] , in which the FO acts to form Ti3+ [4] , and the transparency in UV-Vis region is resultantly improved due to reduction of the absorption originated from VO-related centers [7] . However, the resistivity was limited to 5 × 10−2 Ω∙cm which was too high for TCO, whereas the layers were degenerated by the high electron density [6] . The temperature dependent result also showed that the resistivity was decreased with temperature above 100 K, which indicated influence of the grain-boundaries should be taken into account for the resistivity. Commonly, such feature of resistivity decreased with temperature has been recognized to be due to thermionic emission beyond Schottky-barrier formed in grain-boundary region as demonstrated for poly-crys- talline ZnO layer [8] , in which tunneling of carrier across the boundary-region is expected to dominate at low temperatures. On the other, it is has been well known that anatase-TiO2 shows interesting photo-catalytic reaction and high-hydrophilicity on the surface [9] [10] , which suggests surface is formed with a significantly large amount of surface states. Therefore, the surface trapping electrons maybe also affect the electronic property of the layer.
In this paper, post surface wet-treatment in HCl solution by using metal species of IIIb-group is applied to poly-crystalline LPCVD-TiO2 layers deposited by various conditions to reduce the resistivity with the discussions for sufficient metal-species and carrier transport across the grain-boundaries.
2. Experimental
2.1. TiO2 Deposition by LPCVD
A bell-jar type reactor with the base pressure under 1 × 10−3 Pa evacuated by a combination of diffusion pump and a rotary pump was used for LPCVD of titanium-oxide. Titanium tetra-iso-propoxide (TTIP, Ti(O-i-C3H7)4: 99.7%-purity) purified in vacuum was used as source gas. Details of the apparatus configuration and the purification sequence of TTIP were already shown elsewhere [8] . TTIP vaporized at 65˚C was introduced into the reactor by using a variable valve with monitoring the reactor pressure by Schulz gage. In this work, the pressure of TTIP was fixed at 1.5 × 10−1 Pa during TiO2 deposition. In addition, high purity (99.99999%-purity) H2 gas was also introduced through a mass-flow controller with the flow rate of 4.2 sccm, in which the reactor pressure was 1.0 Pa when the gas was individually introduced into the reactor. TTIP and H2 were simultaneously introduced into the reactor through individual gas inlets and TiO2 layer was deposited at pressure of 2.5 Pa. Niobium pentafluoride powder (NbF5: 98%-purity) used as donor-dopant was charged in a crucible consists of boron-ni- tride (BN) and then thermally evaporated after purification for 5 hrs in vacuum. Evaporation rate of the dopant was estimated from the total evaporation mass evaluated by electronic weight scale after the deposition. Optically flat quartz plate with 1 mm-thick was used as substrate, which was mounted on a substrate holder after chemical cleaning by organic solvents. Temperature of the substrate holder and the BN crucible were increased by resistive heating with controlling by PID-systems using thermo-couples.
2.2. Post Wet-Treatment
Deposited TiO2 layers were dipped in as-purchased conc.-HCl solution (~36% concentration) at room-tempera- ture (RT) after IIIb-group metal (Al, Ga or In: 99.9999%-purity) thin layer was deposited on the TiO2 layer by vacuum evaporation at RT. After the metal layer was sufficiently removed from the surface in the conc.-HCl, in which H2-bubble could not observed on the surface, the layer was dipped again in the conc.-HCl solution without the metal species for 3 minutes. Then, the layer was cleaned in ultra-pure water with the resistivity above 18.2 MΩ∙cm by using Ultra-Sonic bath and dried by a spin-dryer.
2.3. Evaluation
Thickness of the layer was checked by a surface profiler (Veeco, DEKTAK150). Resistivity was evaluated by Van Der Pauw (VDP) method using symmetric four ohmic contacts of indium-dots, in which the temperature was varied from 10 to 320 K in vacuum and dark by a cryogenic system (Janis Research, CCS-150).
3. Results and Discussions
3.1. Temperature Dependence of Resistivity
Figure 1 shows resistivity of as-deposited (open-circle) and wet-treated (solid-circle) TiO2 layer with the thickness about 200 nm at various temperatures ranging from 10 to 320 K, in which the layer was deposited at 380˚C supplying NbF5 and the wet-treatment was performed by using In. It is noted that TiO2 was not etched in the solution and thick metal-layer was not formed on the surface by the treatment because any difference was not observed in the thickness of layer before and after the wet-treatment, further, the result was not dependent on the thickness of the metal layer deposited before dipping in HCl. For the as-deposited layer, the resistivity of 9 × 10−1 Ω∙cm at 320 K was increased with decreasing temperature and saturated about 1.4 Ω∙cm at low temperatures below 100 K. In general, since the carrier mobility of degenerated semiconductor is limited by significant impurity scattering at low temperatures and decreased by photon-scattering at high temperatures with negligible dependence of the carrier density on temperature, it can be considered that resistivity of degenerated semiconductor has little temperature dependence at low temperatures and increases in high temperature region. Therefore, the saturated feature at low temperatures can be recognized the layer is degenerated by the high electron density, however, the result decreasing with temperature above 100 K is reversal to the feature of degenerated semiconductor. Commonly, such result observed above 100 K has been recognized by thermionic carrier conduction over Schottky-barrier in the grain-boundary region as previously demonstrated for poly-crystalline ZnO layer [8] , in which the barrier is formed by captured charges at the traps in the boundaries and the barrier height is owing to the density of the trapped charge in the boundary. In the model for degenerated semiconductor with notably high carrier density, tunneling and thermionic emission across the Schottky-barrier is dominant in the carrier conduction at low and high temperature respectively. In contrast, resistivity of the wet-treated layer was significantly reduced to 2.2 × 10−3 and 3.3 × 10−3 Ω∙cm at 10 and 320 K, respectively, and the temperature dependence saturated at low temperatures and increased with temperature above 100 K was similar to typical feature of degenerated semiconductor. The temperature dependent resistivity of degenerated semiconductor can be discussed on the electron mobility. It has been well recognized that electron mobility in semiconductor influenced by deformation potential scattering due to acoustic phonon and polar scattering due to optical phonon, in which the temperature dependence of mobility resulting from the deformation potential scattering and the polar scattering is T−1.5 and T−0.5 respectively [11] . Since the polar scattering is dominant in polar semiconductor,
![]()
Figure 1. Temperature dependence of resistivity before (as- deposited: open-circle) and after (wet-treated: solid-circle) post wet-treatment, in which the layer was deposited with the thickness about 200 nm at 380˚C in H2-ambient supplying NbF5 with the evaporation rate of 0.05 mg/min.
temperature dependence of the mobility in TiO2 is expected to T−0.5. The resistivity of the wet-treated layer was increased with the temperature according to T0.56 above 100 K, that is, the result suggested the mobility was decreased with temperature by T−0.56 at the temperature region, which was in good agreement to T−0.5 for optical phonon scattered mobility. On the other, some case can be considered for the drastic reduction of the resistivity: 1) if thin metal-layer is formed on the TiO2 layer, the observed resistivity is significantly decreased due to the metal-layer, 2) if the surface Fermi-level is pinned in the conduction band, accumulation layer with high conductivity is formed at the surface, 3) if the interface defects in grain-boundaries are passivated and non-acti- vated by ions, decrease of the Schottky-barrier height resultantly decrease the resistivity. However, these assumptions are denied in the next sections.
3.2. Thickness Dependence of Resistivity
Figure 2 shows resistivity of various thick Nb-F doped TiO2 layers before (as-deposited: open-circle) and after (wet-treated: solid-circle) the wet treatment by using In, where the layers were deposited by same deposition condition except for the deposition period and the resistivity was evaluated at RT. The resistivity of as-deposited layer was gradually decreased with increasing the thickness and saturated above 300 nm. In contrast, the resistivity drastically reduced by the treatment was independent on the thickness thicker than 100 nm, in which the layers showed the resistivity around 3 × 10−3 Ω∙cm. If conductive layer such as metal layer due to residual metal or accumulation layer by surface Fermi-pinning in conduction band is formed at the surface after the wet-treatment, the total conductivity (s) for the layers can be described as s = (ssurf.dsurf. + sbulkdbulk)/d, where ssurf., dsurf., sbulk, dbulk and d is conductivity and thickness of the surface layer, conductivity and thickness of TiO2 layer with the same property of the as-deposited layer, and total thickness of the layer, respectively. The relationship can be approximated to s = ssurf.(dsurf./d) because of ssurf.dsurf. 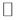 sbulkdbulk, that is, the resistivity ρ is given as ρ = ρsurf.(d/dsurf.) by using the resistivity of surface layer (ρsurf.). Since it can be considered thickness of the surface conductive layer is not dependent on the TiO2 thickness, the resistivity obtained by VDP method is resultantly increased with TiO2 thickness. However, resistivity of the wet-treated layer thicker than 100 nm was scarcely dependent on the TiO2 thickness. The results can be concluded that such conductive surface layer was not formed and the surface or grain-boundary of the layer was modified by the wet-treatment. It should be mentioned here that the resistivity of wet-treated layer with the thickness about 80 nm was higher than that thicker than 100 nm, whereas the resistivity was drastically reduced by the wet-treatment. SEM observations indicated the LPCVD-TiO2 layer consists of the columnar grains. Commonly, columnar grains grow in Volmer-Weber
sbulkdbulk, that is, the resistivity ρ is given as ρ = ρsurf.(d/dsurf.) by using the resistivity of surface layer (ρsurf.). Since it can be considered thickness of the surface conductive layer is not dependent on the TiO2 thickness, the resistivity obtained by VDP method is resultantly increased with TiO2 thickness. However, resistivity of the wet-treated layer thicker than 100 nm was scarcely dependent on the TiO2 thickness. The results can be concluded that such conductive surface layer was not formed and the surface or grain-boundary of the layer was modified by the wet-treatment. It should be mentioned here that the resistivity of wet-treated layer with the thickness about 80 nm was higher than that thicker than 100 nm, whereas the resistivity was drastically reduced by the wet-treatment. SEM observations indicated the LPCVD-TiO2 layer consists of the columnar grains. Commonly, columnar grains grow in Volmer-Weber
![]()
Figure 2. Thickness dependence of resistivity before (as-de- posited: open-circle) and after (wet-treated: solid-circle) post wet-treatment, in which the layers were deposited by same condition (at 380˚C in H2-ambient supplying NbF5 with the evaporation rate of 0.05 mg/min) except for the deposition period.
mode [12] , in which the grains grow via nucleation-step at the initial-stage. Deposition rate of the grain on the nuclear is dependent on the orientation, whereas the orientation of nuclear is not selected on amorphous substrate such as quartz. The grains with orientation bring about the low deposition rate normal to substrate are therefore disappeared on the deposition surface during the deposition because the grains with low deposition rate is covered by the others with high deposition rate, which cause poor crystallinity of layer in thin-region. Thickness of the transient layer is dependent on the deposition condition including the substrate surface and the increased resistivity of the layer thinner than 100 nm is recognized due to the poor crystallinity comparing to that of the thicker layers for the LPCVD.
3.3. Wet-Treatment Using Various IIIb-Group Metal
As shown in the Section 3.1, Indium was so effective to reduce the resistivity of LPCVD-TiO2 layer. In this work, Al and Ga in IIIb-metal were also used for the wet-treatment in addition to the treatment without the metals. Figure 3(a) shows resistivity of 200 nm-thick Nb-F doped layers evaluated at RT before and after the wet- treatment in HCl without and with various IIIb-metal species. The resistivity about 0.1 Ω∙cm of as-deposited layer was not changed by the treatment without the metal-species, which indicated the reduction of resistivity was not due to H and Cl ions. The resistivity was not by using Al but notably reduced by Ga and In, in which In was more effective than Ga. The results clearly showed the reduction of resistivity was dependent on the metal-species. It is noted that such results were not dependent on the thickness of the metal layers deposited on TiO2 layer before the wet-treatment. As well known, electronegativity of IIIb metal is increased with period in the periodic table, in which the value for Al, Ga and In is 1.5, 1.6 and 1.7 respectively [13] . In comparison to the value of 1.5 for Ti, the dependence of resistivity reduction is seemed to be originated from the electronegativity of the metals as shown in Figure 3(b). Here, if the nature of grain-boundaries is modified, such metal ions have to diffuse into the boundaries. It is however difficult to expect that such metal-ions diffuse into the grain-bounda- ries at RT, therefore, it points out that the surface of layer was modified by the metal-species to reduce the Schottky barrier height in the grain-boundary. As the result, it can be concluded that not only the nature of grain-boundary but also surface states of the layer should be taken into account to disclose the carrier conduction across the boundary. Especially, the influence of surface states may be notable in anatase-TiO2 because surface defects able to capture electrons is easily formed in the oxide, which is resulted in highly photo-chemical reactions at the surface [9] [10] . Analysis of the surface chemical states is in progress with the modeling for carrier transport across the grain-boundaries as below. When electrons are captured to the surface states which are probably formed by contribution surface oxygen of the layer, Fermi-level at the surface is pinned at deeper level in the forbidden band and the energy-band at the surface is bent to upper in electron energy. The influence of
![]()
![]() (a) (b)
(a) (b)
Figure 3. Resistivity of 200 nm-thick layers treated in HCl without and with IIIb-metal in addition to that of the as-deposited layer, where the layers were deposited at 380˚C by NbF5 evaporation rate of 0.05 mg/min and the treatment was individually carried out for each layers.
band-bending for the resistivity is negligible in the kernel (region without the influence of grain-boundary) with so high electron-density but should be taken into account in the grain-boundary region where the donor is compensated by defects due to the structural-disordering. If the surface states are modified to shallower level by the metal-ions with high electronegativity, the captured electron at the surface is decreased in the density, which brings about decrease of band-bending near the surface including grain-boundary. Further, such results indicated that other metal species with larger electrogenativity might be useful for the treatment instead of In.
3.4. Dependence of Resistivity on Deposition Condition
Since it is considered resistivity of the layer was increased by the insufficient influence originated from the grain-boundaries and the surface states as discussed in Section 3.2 and 3.3, the results after the wet-treatment using In indicated the optimum deposition condition to reduce resistivity of the kernel. Figure 4(a) and Figure 4(b) show resistivity evaluated at RT of as-deposited (open-circle) and wet-treated (solid-circle) layer for the deposition temperature and NbF5 evaporation rate from crucible respectively, where the layers were deposited with the thickness about 200 nm. The resistivity of layer was decreased with deposition temperature and increased at 400˚C for the as-deposited layer as shown in Figure 4(a), where NbF5 was supplied with the evaporation rate of 0.05 mg/min from crucible. The resistivity drastically reduced by the wet-treatment was also decreased with temperature but the optimum temperature around 370˚C to fabricate low resistive layer was clearly lower than that for the as-deposited layer. It is considered from the result, the low resistive kernel was successfully formed around 370˚C but higher temperature was required to improve the grain-boundaries and/or the surface. We previously speculated the reduction of resistivity was occurred by the reduction of anti-site Nb in the density [7] . The result after the wet-treatment suggested the donor-compensating defect was easily formed in the grain-boundaries, which was probably related to the dissociation scheme of TTIP in the boundaries. On the other, the resistivity of as-deposited layer was decreased with NbF5 evaporation rate and increased by excessive supply of the dopant above 0.6 mg/min as shown in Figure 4(b), where the layers were deposited at 380˚C. The notably decreased resistivity by the wet-treatment was also dependent on NbF5 evaporation rate but the optimum supply rate around 0.2 mg/min to reduce the resistivity was clearly low in comparison to that for the as-deposited layer. The lager optimum NbF5 evaporation rate for the as-deposited layer indicated NbF5 was also employed to improve the property of grain-boundaries in addition to the donor-doping into the kernel. In previous results for the deposition, it was concluded that F on the deposition surface promotes dissociation of TTIP, which is resulted in reduction of oxygen-vacancies, but excess F disturbed the dissociation due to fluorination of TTIP in vapor phase. Since the resistivity of wet-treated undoped layer was comparable to that of wet-treated doped layer, it is considered that the property of grain-boundary was improved by F during the deposition. It is noted that although the low resistive TiO2 layer can be fabricated in H2-ambient without the doping, the doping is necessary
![]()
![]() (a) (b)
(a) (b)
Figure 4. Resistivity of 200 nm-thick layers treated in HCl without and with IIIb-metal in addition to that of the as-deposited layer, where the layers were deposited at 380˚C by NbF5 evaporation rate of 0.05 mg/min and the treatment was individually carried out for each layers.
to increase the transparency in UV-Vis region by removal oxygen-deficiency in the layer as reported previously [7] .
4. Conclusion
Post wet-treatment for LPCVD-TiO2 layer was demonstrated in HCl by using IIIb-metal. The resistivity of as-deposited layer was drastically reduced after the post-treatment by using In. The temperature dependence of resistivity indicated that the layer was degenerated and Schottky-barrier was formed in the grain-boundary of the as-deposited layer but the barrier was not observed for the wet-treated layer. The result for the wet-treated layer was recognized to be originated from the improved surface of layer by metal-species, in which the metal with higher electronegativiy comparing to Ti was more effective to reduce the resistivity. The resistivity for the as- deposited and the wet-treated layer deposited by various conditions suggested that the optimum condition to reduce the resistivity of layer was different for the grain and the grain-boundary. For example, higher deposition temperature and excessive NbF5 supply were required to improve the property of the grain-boundary comparing to that to fabricate the low resistive grain. As the result after removal influence of the grain-boundary by the wet-treatment, it was clarified that TiO2 layer with the resistivity as low as 3 × 10−3 Ω∙cm can be fabricated by the LPCVD. Such results in this work point out that lower resistive LPCVD-TiO2 layer able to apply for transparent conductive oxide can be fabricated by successful control of the grain-boundary and the surface property.
NOTES
*S. Hatakeyama now belongs to Hitachi Industry Solution Ltd.