Kinetic and Equilibrium Isotherms Studies of Adsorption of Pb(II) from Water onto Natural Adsorbent ()
1. Introduction
Environmental pollutants can be treated by chemical, biological and/or physical processes [1] - [6] . Adsorption process is one of the physical treatment methods. In this technique, adsorbate molecules accumulated onto the surface of adsorbent. It is known that water polluted with low concentration or even trace levels of heavy metals can cause serious health problems to human body [5] . Recently, one of the critical reviews on current treatment methods of removal of heavy metals [7] reported that adsorption was an effective method and was neither costly nor required various tools. In adsorption process the most effective adsorbent was reported to be activated carbon (AC) which used for the removal many pollutants, including heavy metals [2] . However, production of activated carbon by carbonizing and activating the organic substances from the proper materials is highly cost, and thus requires the search for alternatives and low-cost adsorbents [8] . A low coast adsorbent should be inexpensive materials and does not require many processing. Furthermore, it can be found in the environment naturally in a plentiful or produced as a by-product or a waste from an industry or agriculture [9] . Several low-cost adsorbents using leaf powder of different trees such as bael tree [10] , cypress, cinchona and pine [11] , neem [2] , rubber [12] , Cinnamomum camphora [13] , castor [14] , Solanum melongena [15] , and others were used for removing Pb(II) ions from aqueous solution. To make further use of leaf powder of trees, the present study is an attempt to use Dobera glabra Forssk leaves (DL), as nonconventional low-cost adsorbent for removal of some heavy metal ions such as Pb(II), and Ni(II) ions from aqueous solution. Dobera tree (an ever-green tree), is a member of the Salvadoraceae family and presents in plentiful in many countries such as Saudi Arabia. It is characterized by alternate thick skinny leaves [16] [17] . In the current study, kinetics of Pb(II) adsorption onto DL studied through a pseudo-first-order [18] - [20] , a pseudo-second-order [21] [22] and an intraparticle diffusion [18] [23] . Adsorption isotherm (adsorption equilibrium) is usually described the equilibrium state between the amount of adsorbed metal ion onto the adsorbent surface 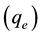 and the concentration of metal ions in solution
and the concentration of metal ions in solution 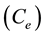 at a fixed both temperature and pH. The common equations used to describe adsorption isotherm are Langmuir [24] [25] and Freundlich [25] [26] in addition to Temkin [27] - [29] and Dubinin-Radushkevich (D-R) equation [30] [31] . The parameters of those equations can describe both the surface properties and the affinity of the adsorbent such as DL to the adsorbate such as metal ions. Also, adsorption maximum capacity, adsorption intensity and the heat of adsorption can be determined. In this study, the linear forms of the above mentioned equations were used to describe the equilibrium data hence their applicability was evaluated by determining the correlation coefficients (R2) [31] .
at a fixed both temperature and pH. The common equations used to describe adsorption isotherm are Langmuir [24] [25] and Freundlich [25] [26] in addition to Temkin [27] - [29] and Dubinin-Radushkevich (D-R) equation [30] [31] . The parameters of those equations can describe both the surface properties and the affinity of the adsorbent such as DL to the adsorbate such as metal ions. Also, adsorption maximum capacity, adsorption intensity and the heat of adsorption can be determined. In this study, the linear forms of the above mentioned equations were used to describe the equilibrium data hence their applicability was evaluated by determining the correlation coefficients (R2) [31] .
2. Experimental
2.1. Chemicals
Analytical-grad chemicals were used in this work without further purification. To avoid any interference of other ions, all solutions were prepared using HPLC water. To evaluate the significant of the adsorbent, stock solutions (1000 mg/L) of Pb(II) was prepared from their nitrate salts (purchased from Sigma) and further diluted to perform adsorption experiments. To study the selectivity of the adsorbent, preliminary adsorption experiments performed by mixing nitrate solutions of both Pb(II) and Ni(II) in presence of dobera leaves. Dilute solutions of 1 M HNO3 (HPLC grad) and 1 M NaOH (BDH) were used to adjust pH of metal ion solutions using a pH meter.
2.2. Adsorbent
Dobera leaves (DL) was used as a natural adsorbent. DL were collected from fields around El-Ardh area (Bathan) in Jazan, Saudi Arabia. To remove dust and any other impurities present on the leaves, adsorbent was air dried and washed several times with distilled water. Powder of DL was formed by drying the leaves then ground well and sieved.
2.3. Adsorption Experiments
Pb(II) solution was used in this study as an environmental pollutants to evaluate the significant of the adsorbent. Stock solutions (1000 mg/L) of Pb(II) was prepared from their nitrate salts using HPLC water and further diluted to perform adsorption experiments. Preliminary adsorption selectivity experiment performed by mixing both Pb(II) and Ni(II) solutions. Calibration curves were prepared by serial dilutions (1.0 to 10.0 mg/L). In batch experiments, known amounts of DL were added into several 50 mL conical flasks, each containing 25.0 mL solution of Pb(II) and/or Ni(II) with an initial concentration ranging from 5.0 to 50 mg/L. Then the flasks were shaked at 120 rpm using an electric shaker at room temperature. Samples were withdrawn at interval times and a laboratory table centrifuge was used to separate DL powder from samples. Adsorption isotherms were determined by introducing 0.025 g (1.0 g/L) DL powder to respective 25.0 mL solution of different Pb(II) concentrations (5 - 50 mg/L) at room temperature.
2.4. Effect of Adsorbent (DL Powder) Mass
To investigate the effect of DL powder mass, different mass of DL 0.25 to 2 g/L (0.00625 - 0.05 g/0.025L) were introduced to a number of conical flasks containing a specific volume of a fixed [Pb(II)]0 at the same pH and room temperature. Concentrations of Pb(II) were measured at equilibrium.
2.5. Analytical Methods
Inductively coupled plasma optical emission spectrometry (ICP-OES) was used to determine the concentrations of both Pb(II) and Ni(II). These concentration measurements lead to obtain a calibration curves which used to calculate metal ion concentrations during adsorption experiments at any time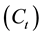 .
.
The amount of metal ion sorbed onto DL powder at any time, 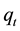 , was calculated from:
, was calculated from:
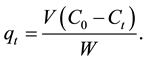 (1)
(1)
At equilibrium, 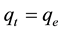 and
and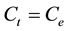 ; therefore the amount of sorbed metal ion,
; therefore the amount of sorbed metal ion, 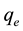 , was calculated from
, was calculated from
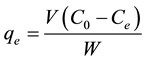 (2)
(2)
where 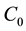 ,
, 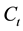 and
and 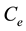 are the initial concentration, concentration at any time and equilibrium concentrations of metal ion solution (mg/L), respectively, V is the volume of the solution (L), and W is the mass of DL powder (g) [28] . Metal ion removal percentage can be calculated as follows:
are the initial concentration, concentration at any time and equilibrium concentrations of metal ion solution (mg/L), respectively, V is the volume of the solution (L), and W is the mass of DL powder (g) [28] . Metal ion removal percentage can be calculated as follows:
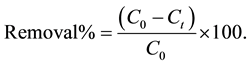 (3)
(3)
3. Results and Discussion
3.1. Effects of Contact Time
A dobera leaves (DL) dosage of 0.025 g (1.0 g/L) was added to 0.025 L of 30 mg/L of Pb(II) solution. Experiments were conducted at a temperature of 298 K for 120 min to test the effect of contact time on the adsorption process. The results (Figure 1) indicated that the adsorption of Pb(II) onto DL was very rapid in the first 15 min hence the adsorbed amount (qt) reached 24.93 mg/L, which represented about 83%, comparing to 29.76 mg/g
![]()
Figure 1. Effect of contact time on the adsorption of Pb(II) onto dobera leaves (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
(99.2%) at 180 min. Then the adsorption of Pb(II) ions increased gradually during the following 45 min until reached equilibrium at about 150 min. The results showed that the uptake of Pb(II) ions by DL depends on contact time. This may be due to the time required for the Pb(II) to encounter the boundary layer effect, then diffuse to the surface of DL and finally diffuse to the porous structure of the adsorbent [32] . To ensure complete equilibrium of the data, adsorption samples were collected at 180 min.
3.2. Effect of Solution pH on Pb(II) Removal
It was found that initial pH has a great effect on the rate of adsorption by affecting speciation and the degree of ionization of adsorbate in addition to the surface charge of adsorbent [33] [34] .
Thus experiments were conducted at 30 mg/L [Pb]0, 1.0 g/L DL dose, and 180 min contact time at a temperature of 298 K, to study the effect of solution pH on the equilibrium adsorption capacity (qe) of Pb(II) onto DL as shown in Figure 2. It is indicated that % removal of Pb(II) reached a maximum in a slight acidic medium and decreased in slight basic medium. The % removal of Pb(II) increased from 92% at pH 3 to maximum, 95%, at pH 5. While decreased from 87% at pH 6 to reach 78.44% by increasing the pH values to 8. Therefore, further adsorption experiments were performed at pH 5 as an optimum pH value. It was reported that the predominant species of Pb(II) are Pb2+ ions at pH between 5 and 6 [33] . However, in presence of high concentration of H+ ions at pH 3, hydronium ions H3O+ ions would compete with Pb2+ ions for the binding sites, leading to lower the % removal of Pb2+ ions. While decreasing the concentration of H3O+ ions at pH 5 leads to decrease the positive charge on the surface of DL which lower the electrostatic repulsion between the surface of DL and Pb(II) hence increased % removal to 95%. A similar trend was previously reported by the adsorption of Pb(II) onto tobacco stem [24] and some metal cations onto different adsorbents [35] . Also, adsorption of Pb2+, Cd2+ and Ni2+ reached maximum biosorption at pH 5.5 using chemically modified orange peel [26] . On the other hand, increasing the pH to 8 would increase the concentration of OH− ions leading to the formation of the predominant species of Pb(OH)2. Due to the low solubility of Pb(OH)2 at 298 K, the concentration of Pb2+ ions would decrease at pH 8 mainly due to precipitation, thus adsorption process has no meaning at pH 8 for Pb2+ ions [36] . These results can be further proven by the results in our previous study for the adsorption of methylene blue dye, a cationic dye (MB dye+), onto miswak leaves, [28] . The adsorption rate found to decrease in high acidic medium at pH 2.8 due the effective compete between MB dye+ and H+ ions for binding sites.
3.3. Effect of Adsorbed Amount
At constant [Pb(II)]0 (30 mg/L), different amounts of DL (0.25 to 2.0 g/L) were added to metal ion solutions (0.025 L) to study the effect of DL amount on Pb(II) adsorption. Results in Figure 3 shows that the adsorption capacity in the first stage increased rapidly with the increase in the adsorbent dose then increased slowly until reached equilibrium with the further increase in the adsorbent dose. It can be seen that at 1.0 g/L of the adsorbent dose, the % removal of Pb(II) reached the most at 94.8%. Then an increase in the dose of DL from 1.0 to 2.0 g/L resulted only in about 3% more to reach 97.1%. Thus 1.0 g/L of DL was chosen as the optimum dose and used in the further experiments. The increase in % removal of Pb(II) with the increase in the amount of DL
![]()
Figure 2. Effect of solution pH on the adsorption of Pb(II) and Ni(II) ions onto dobera (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, V = 0.025 L, DL dosage = 1.0 g/L).
![]()
Figure 3. Effect of adsorbent dose on the adsorption of Pb(II) onto dobera (T = 298 K, time =180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 - 2.5 g/L).
up to 1.0 g can be assigned to the increase in both the surface area and the adsorption sites to Pb(II). However, the adsorption rate did not enhanced effectively by increasing the amount of the adsorbent from 1.0 to 2.0 g/L, may be due to increase the overlapping and/or aggregation of adsorbent sites at high dose [37] . Effect of dose of Rosa bourbonia waste phyto-biomass which was used for the adsorption of Pb(II) and Cu(II) from aqueous media indicated similar trend. Hence the dose of 2 g/L was the optimum when the dose of adsorbent was varying from 2.0 to 5.0 g/L [37] .
3.4. Adsorption Kinetics
Fitting the experimental data into different kinetic models enables to study the adsorption rate, model the process and predict information about adsorbent/adsorbate interaction (physisorption or chemisorption) [14] . In this study, three different models were used such as the pseudo-first-order [18] - [20] , the pseudo-second-order [21] [22] and intraparticle diffusion [18] [23] .
3.4.1. Pseudo-First-Order Equation
Pseudo-first-order equation was given by Langergren and Svenska (1898) to determine the rate constant of adsorption process as:
![]() (4)
(4)
where ![]() and
and ![]() are the amounts of the Pb(II) adsorbed (mg/g) at equilibrium and at time t (min), respectively, and
are the amounts of the Pb(II) adsorbed (mg/g) at equilibrium and at time t (min), respectively, and ![]() is the rate constant of adsorption (min−1). Values of
is the rate constant of adsorption (min−1). Values of ![]() and
and ![]() were calculated from the slope and the intercept of the plots of
were calculated from the slope and the intercept of the plots of ![]() versus
versus ![]() respectively at different concentrations (Figure 4). The results in Table 1 show that the values of R2 were low and the experimental
respectively at different concentrations (Figure 4). The results in Table 1 show that the values of R2 were low and the experimental ![]() value does not agree well with the calculated value. This shows that the adsorption of Pb(II) onto DL is not first-order kinetics.
value does not agree well with the calculated value. This shows that the adsorption of Pb(II) onto DL is not first-order kinetics.
3.4.2. Pseudo-Second-Order Rate Equation
Equation of pseudo-second-order based on equilibrium adsorption can be expressed as:
![]() (5)
(5)
or
![]() (6)
(6)
where ![]() (g/mg・min) is the adsorption rate constant of pseudo-second-order adsorption rate. The value of
(g/mg・min) is the adsorption rate constant of pseudo-second-order adsorption rate. The value of ![]() and
and ![]() can be obtained from the slope and the intercept of the plot of
can be obtained from the slope and the intercept of the plot of ![]() versus
versus ![]() respectively. The results
respectively. The results
![]()
Figure 4. Pseudo-first-order kinetics for the adsorption of Pb(II) onto dobera (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
![]()
Table 1. Adsorption kinetic parameters for the adsorption of Pb(II) onto dobera (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
in Figure 5 show linear plots with very high values of R2 (Table 1) in addition to the good agreement between experimental and calculated values of ![]() . Therefore, the adsorption of Pb(II) onto DL is greatly represented by the pseudo-second-order kinetics. The applicability of second-order to the adsorption data of Pb(II) onto DL indicates that the concentration of both DL and Pb(II) ions are involved in the rate determining step and the adsorption process may be chemisorption. Similar trends were shown for the adsorption of Pb(II) onto Tobacco stem [24] and castor leaf powder [14] .
. Therefore, the adsorption of Pb(II) onto DL is greatly represented by the pseudo-second-order kinetics. The applicability of second-order to the adsorption data of Pb(II) onto DL indicates that the concentration of both DL and Pb(II) ions are involved in the rate determining step and the adsorption process may be chemisorption. Similar trends were shown for the adsorption of Pb(II) onto Tobacco stem [24] and castor leaf powder [14] .
3.4.3. Intra-Particle Diffusion Study
In order to investigate the mechanism of Pb(II) adsorption onto DL, intra-particle diffusion based mechanism was studied. It is proposed that the uptake of the adsorbate (Pb(II)) by the adsorbent (DL) varies almost proportionately with the square root of the contact time (t1/2) according to the following equation [13] .
![]() (7)
(7)
where, ![]() is the amount of Pb ion adsorbed per unit mass of adsorbent (mg/g) at a time
is the amount of Pb ion adsorbed per unit mass of adsorbent (mg/g) at a time ![]() and
and ![]() the intra-particle diffusion rate constant (mg/g・min−1/2). The rate constant
the intra-particle diffusion rate constant (mg/g・min−1/2). The rate constant ![]() of stage i is obtained from the slope of the straight line of
of stage i is obtained from the slope of the straight line of ![]() versus
versus ![]() . The results indicated that the plot in Figure 6 was not linear over the whole
. The results indicated that the plot in Figure 6 was not linear over the whole
time range. Also, the plot indicated that the external surface adsorption (stage 1) is absent and it is finished before 15 min. Then after 15 min, the intra-particle diffusion control (stage 2) started and kept on to 60 min. Finally, the equilibrium step (stage 3) appeared after 60 min. It can be seen that the adsorption of Pb ion onto DL involved more than one process, and the intra-particle transport is not the rate-limiting step.
3.5. Isotherms for the Sorption of Pb(II) onto Dobera Leaves
Adsorption isotherms are different models used to evaluate the affinity of studied adsorbent (DL) for the removal of Pb(II) from aqueous solution. In the current study, four isotherm models namely Langmuir, Frendulich, Temkin and D-K were used.
![]()
Figure 5. Pseudo-second-order kinetics for the adsorption of Pb(II) onto dobera (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
![]() t (min1/2)
t (min1/2)
Figure 6. Intra-particle diffusion plot for the adsorption of Pb(II) onto dobera (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
3.5.1. Langmuir Isotherm
The Langmuir isotherm model assumes that a monolayer of adsorbed material (in liquid, such as Pb(II)) is adsorbed over a uniform adsorbent surface such as DL. The Langmuir-II equation is derived by some mathematical manipulation as:
![]() (8)
(8)
where ![]() (the maximum capacity of adsorption, mg/g) and
(the maximum capacity of adsorption, mg/g) and ![]() (a constant related to the affinity of the binding sites, L/mg) are the Langmuir isotherm constants. Both
(a constant related to the affinity of the binding sites, L/mg) are the Langmuir isotherm constants. Both ![]() and
and ![]() can be determined from the linear form Langmuir-II equation as:
can be determined from the linear form Langmuir-II equation as:
![]() (9)
(9)
Figure 7 shows the linear fit of Langmuir-II for the adsorption of Pb(II) onto DL at 25˚C is. The value of ![]() ,
, ![]() and
and ![]() are presented in Table 2. The high value of
are presented in Table 2. The high value of ![]() as 0.999 indicated minimal deviation from the fitted equation showing that the adsorption data would follow Langmuir equation [2] . Also, the data in Table 2 indicated that the maximum adsorption capacity of DL for Pb(II) was calculated as 83 mg/g. It can be mentioned that the surface of DL is homogeneous and the adsorption of Pb(II) formed a monolayer on its outer surface [25] . N. Feng et al. [26] also, found the adsorption of some heavy metals such as Pb(II) onto chemically modified orange peel (OPAA) followed Langmuir model and formed monolayer. Other authors have reported a monolayer capacity (
as 0.999 indicated minimal deviation from the fitted equation showing that the adsorption data would follow Langmuir equation [2] . Also, the data in Table 2 indicated that the maximum adsorption capacity of DL for Pb(II) was calculated as 83 mg/g. It can be mentioned that the surface of DL is homogeneous and the adsorption of Pb(II) formed a monolayer on its outer surface [25] . N. Feng et al. [26] also, found the adsorption of some heavy metals such as Pb(II) onto chemically modified orange peel (OPAA) followed Langmuir model and formed monolayer. Other authors have reported a monolayer capacity (![]() ) of 33.78 mg/g at 25˚C for the adsorption of Pb(II) by electric furnace slag [5] . In addition, removal of Cr(IV) by adsorption onto eight different natural adsorbent formed a monolayer and followed Langmuir isotherm as reported by B. Singha et al. [27] . On the other hand, comparison of maximum monolayer adsorption capacity
) of 33.78 mg/g at 25˚C for the adsorption of Pb(II) by electric furnace slag [5] . In addition, removal of Cr(IV) by adsorption onto eight different natural adsorbent formed a monolayer and followed Langmuir isotherm as reported by B. Singha et al. [27] . On the other hand, comparison of maximum monolayer adsorption capacity ![]() of Pb(II) onto various adsorbents obtained in the literature is presented in Table 3 in order to compare the efficiency of dobera leaves (DL). It can be seen that DL is very effective adsorbent for Pb(II) with a relatively large adsorption capacity of 83 mg/g when compared with some other adsorbents.
of Pb(II) onto various adsorbents obtained in the literature is presented in Table 3 in order to compare the efficiency of dobera leaves (DL). It can be seen that DL is very effective adsorbent for Pb(II) with a relatively large adsorption capacity of 83 mg/g when compared with some other adsorbents.
![]()
Figure 7. Langmuir isotherm for the adsorption of Pb(II) onto dobera leaves (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
![]()
Table 2. Langmuir, Freundlich, Temkin and D-R constants for the adsorption of Pb(II) onto dobera leaves (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
![]()
Table 3. Comparison of the maximum monolayer adsorption of Pb(II) onto various adsorbents.
3.5.2. Separation Factor
Langmuir isotherm can be characterized by a dimensionless constant called separation factor![]() . [5] [28] as shown by the following equation:
. [5] [28] as shown by the following equation:
![]() (10)
(10)
where ![]() (mg/L) is the highest [Pb(II)]0 and
(mg/L) is the highest [Pb(II)]0 and ![]() (L/mg) is Langmuir constant. The value of
(L/mg) is Langmuir constant. The value of ![]() indicates the shape of the isotherm to be either linear
indicates the shape of the isotherm to be either linear![]() , unfavourable
, unfavourable![]() , favourable
, favourable![]() , or irreversible
, or irreversible![]() . Figure 8 representing the Plot of
. Figure 8 representing the Plot of ![]() versus
versus ![]() of Pb(II) at 25˚C. It can be seen that the
of Pb(II) at 25˚C. It can be seen that the
![]() values were in the range of 0. 01 to 0.09, which is less than unity, indicating that the adsorption of Pb(II) onto DL is a favourable process and thus DL is, a good adsorbent for Pb(II).
values were in the range of 0. 01 to 0.09, which is less than unity, indicating that the adsorption of Pb(II) onto DL is a favourable process and thus DL is, a good adsorbent for Pb(II).
3.5.3. Surface Coverage (θ)
Another factor can help for understanding the behavior of the adsorption of Pb(II) onto DL is the Langmuir type equation which related the surface coverage (θ) of the adsorbent (DL) to the initial concentration of Pb(II) ![]() as follow:
as follow:
![]()
Figure 8. Plot of separation factor versus initial Pb(II) concentration for the adsorption of Pb(II) onto dobera leaves (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
![]() (11)
(11)
![]() (12)
(12)
where ![]() is Langmuir constant (the adsorption coefficient),
is Langmuir constant (the adsorption coefficient), ![]() is the initial concentration of Pb(II) ions and
is the initial concentration of Pb(II) ions and ![]() is the surface coverage. The results in Figure 9 show that the adsorption of Pb(II) onto DL was very fast in the beginning. Also, the surface coverage increases rapidly with the increase of initial Pb(II) concentration, and then increases slowly when the initial concentration exceeds 30 mg/L until θ value is close to 1.0. This results show that DL will be very effective adsorbent in removing Pb(II) ions from aqueous solutions.
is the surface coverage. The results in Figure 9 show that the adsorption of Pb(II) onto DL was very fast in the beginning. Also, the surface coverage increases rapidly with the increase of initial Pb(II) concentration, and then increases slowly when the initial concentration exceeds 30 mg/L until θ value is close to 1.0. This results show that DL will be very effective adsorbent in removing Pb(II) ions from aqueous solutions.
3.5.4. Freundlich Isotherm
The Freundlich exponential equation presumes that the adsorption process takes place on a heterogeneous surface. Both nonlinear and linear forms of Freundlich are given as:
![]() (13)
(13)
![]() (14)
(14)
where ![]() (L/mg) is an indicator of the multilayer adsorption capacity and
(L/mg) is an indicator of the multilayer adsorption capacity and ![]() is the adsorption intensity and
is the adsorption intensity and
indicates both the relative distribution of energy and the heterogeneity of the adsorbent sites.
Figure 10 representing the linear plot of ![]() versus
versus ![]() at constant temperature. The value of
at constant temperature. The value of ![]() and
and ![]() (Table 2) were determined from the intercept and the slope respectively. Although, the value of
(Table 2) were determined from the intercept and the slope respectively. Although, the value of ![]()
(0.981) of Freundlich is slightly lower than the value of ![]() (0.999) of Langmuir-II isotherm. The value of
(0.999) of Langmuir-II isotherm. The value of ![]() (indicative of favorability when
(indicative of favorability when![]() ) is 0.706, which is close to the unity and indicates the favorability of the adsorption process. Therefore, Freundlich model is still a good model to describe the adsorption data. The results of Langmuir and Freundlich implies that the adsorption of Pb(II) onto DL show a complex mechanism involving both monolayer and heterogeneous surface condition. Previous reports indicated that adsorption of Pb(II) onto different biosorbents such as Tobacco stem [24] and the Neem (Azadirachta indica) leaves show similar results [5] .
) is 0.706, which is close to the unity and indicates the favorability of the adsorption process. Therefore, Freundlich model is still a good model to describe the adsorption data. The results of Langmuir and Freundlich implies that the adsorption of Pb(II) onto DL show a complex mechanism involving both monolayer and heterogeneous surface condition. Previous reports indicated that adsorption of Pb(II) onto different biosorbents such as Tobacco stem [24] and the Neem (Azadirachta indica) leaves show similar results [5] .
3.5.5. Temkin Isotherms
The adsorption potential of adsorbent to adsorbate can be tested by applying Temkin isotherm model (Equation
![]()
Figure 9. Surface coverage (θ) for the adsorption of Pb(II) onto dobera leaves at different concentrations (T = 298 K, time = 180 min, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
![]()
Figure 10. Freundlich isotherm for the adsorption of Pb(II) onto dobera leaves (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
(9)). This equation supposes that increasing the coverage layer of adsorbate onto the surface of adsorbent makes the heat of adsorption (DHads) of all molecules in that layer decreased linearly by increase the coverage. The linear form of Temkin is:
![]() (15)
(15)
where, R is common gas constant (0.008314 kJ/mol・K), T is the absolute temperature (K), ![]() is the Temkin constant related to the heat of sorption (kJ/mol) which indicates the adsorption potential (intensity) of the adsorbent and
is the Temkin constant related to the heat of sorption (kJ/mol) which indicates the adsorption potential (intensity) of the adsorbent and ![]() (L/g) is Temkin constant related to adsorption capacity. The constants
(L/g) is Temkin constant related to adsorption capacity. The constants ![]() and
and ![]() can be calculated from the liner plots of
can be calculated from the liner plots of ![]() versus
versus ![]() as shown in Figure 11. The numerical values of correlation coefficient,
as shown in Figure 11. The numerical values of correlation coefficient, ![]() in addition to both
in addition to both ![]() and
and ![]() of the Temkin equation for Pb(II) is represented in Table 2. Due to the low value of
of the Temkin equation for Pb(II) is represented in Table 2. Due to the low value of ![]() , (0.974), the data of equilibrium isotherms of Pb(II) onto DL is poorly described by the Temkin model. Also, the slight fitting of Temkin isotherm model for the adsorption of Pb(II) ions by Anethum graveolens was reported [18] .
, (0.974), the data of equilibrium isotherms of Pb(II) onto DL is poorly described by the Temkin model. Also, the slight fitting of Temkin isotherm model for the adsorption of Pb(II) ions by Anethum graveolens was reported [18] .
3.5.6. Dubinin-Radushkevich (D-R) Isotherm Model
To Calculation the Sorption Energy, D-R model was used. This isotherm dose not assume constant adsorption potential or homogeneous surface for the adsorbent [1] . Thus the D-R linear form (Equation (10)) can be applied on both homogenous and heterogeneous surfaces [13] .
![]() (16)
(16)
where, ![]() , a constant related to the mean free energy of adsorption
, a constant related to the mean free energy of adsorption![]() ,
, ![]() (mg/g) is the theoretical
(mg/g) is the theoretical
![]()
Figure 11. Temkin isotherm for the adsorption of Pb(II) onto dobera leaves (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
saturation capacity based on D-R isotherm and ![]() is the Polanyi potential.
is the Polanyi potential. ![]() can be calculated from the following equation:
can be calculated from the following equation:
![]() (17)
(17)
where R![]() , is the gas constant and T (K) is the absolute temperature. Figure 12 representing the plot of
, is the gas constant and T (K) is the absolute temperature. Figure 12 representing the plot of ![]() against
against ![]() for adsorption of Pb(II) onto DL which allows for the determining
for adsorption of Pb(II) onto DL which allows for the determining ![]() and
and ![]() from the intercept and the slope respectively. Table 2 shows the numerical values of
from the intercept and the slope respectively. Table 2 shows the numerical values of ![]() and the correlation coefficient (R2). The high value of R2 indicated that the adsorption of Pb(II) onto DL followed D-R isotherm model.
and the correlation coefficient (R2). The high value of R2 indicated that the adsorption of Pb(II) onto DL followed D-R isotherm model.
Mean energy of adsorption (Ea):
Applying D-R isotherm model for the adsorption data of Pb(II) onto DL allows to calculate the mean energy of adsorption ![]() from the value of
from the value of ![]() according to the following relation:
according to the following relation:
![]() (18)
(18)
The numerical value of ![]() (Table 2) was calculated as
(Table 2) was calculated as![]() . That means the free energy change required when one mole of Pb(II) ions adsorbed from infinity in the solution onto the surface of DL is equal to
. That means the free energy change required when one mole of Pb(II) ions adsorbed from infinity in the solution onto the surface of DL is equal to![]() . According to the literature [13] , when the value of
. According to the literature [13] , when the value of ![]() is in the range of
is in the range of ![]() indicates physical adsorption process.
indicates physical adsorption process.
3.6. Standard Gibbs Free Energy Change (DG˚)
Standard free energy ![]() can be calculated from the following equations [2] ;
can be calculated from the following equations [2] ;
![]() (19)
(19)
![]() (20)
(20)
where, ![]() is the temperature (K),
is the temperature (K), ![]() is gas constant (kJ/mol・K) and
is gas constant (kJ/mol・K) and ![]() (L/g) is the standard thermodynamic equilibrium constant,
(L/g) is the standard thermodynamic equilibrium constant, ![]() is the amount of adsorbed Pb(II) per unit mass of DL at equilibrium (29.755 mg/g) and
is the amount of adsorbed Pb(II) per unit mass of DL at equilibrium (29.755 mg/g) and ![]() is the equilibrium aqueous concentration of Pb(II) (0.245 mg/L). Applying Equation (17) resulted in negative value (−11.89 kJ/mol) of
is the equilibrium aqueous concentration of Pb(II) (0.245 mg/L). Applying Equation (17) resulted in negative value (−11.89 kJ/mol) of ![]() indicating that the adsorption process of Pb(II) onto DL is spontaneous.
indicating that the adsorption process of Pb(II) onto DL is spontaneous.
3.7. Preliminary Selectivity Study of Pb(II)
Preliminary experiments were conducted to investigate the selectivity of Pb(II) from the mixture of solution with same charge. Only one mixture was tested such as Pb(II)/Ni(II). The following equations [38] were used to calculate the distribution coefficient ![]() (mL/g) and the selectivity coefficient k,
(mL/g) and the selectivity coefficient k,
![]() (21)
(21)
![]() (22)
(22)
where ![]() and
and ![]() represent the initial and equilibrium concentrations of the given metal ions in solution, respectively.
represent the initial and equilibrium concentrations of the given metal ions in solution, respectively. ![]() and
and ![]() represent the distribution coefficient of Pb(II) and Ni(II) ions respectively.
represent the distribution coefficient of Pb(II) and Ni(II) ions respectively.
The results indicated that the value of ![]() for Pb(II) was very large (1874.3 mL/g) comparing to
for Pb(II) was very large (1874.3 mL/g) comparing to ![]() for Ni(II) which was equal to 9.72 mL/g. Therefore, calculating the value of selectivity coefficient (k) from Equation (19) resulted as 235.5 and 0.005 for Pb(II) and Ni(II) respectively showing the higher selectivity of DL to Pb(II) ions.
for Ni(II) which was equal to 9.72 mL/g. Therefore, calculating the value of selectivity coefficient (k) from Equation (19) resulted as 235.5 and 0.005 for Pb(II) and Ni(II) respectively showing the higher selectivity of DL to Pb(II) ions.
3.8. Removal of Pb(II) from Drinking Water
Experiments were conducted to test removal of Pb(II) onto DL from drinking water. Different samples of drinking water from Jazan area, KSA, were collected from different sources such as Sadeem, Mahlia, Arghad, Areedah, Aswar, Alwasm and Abu-Ziab. All samples were undergoes the optimum conditions of adsorption batch experiments as discussed previously. The results in Figure 13 show that the % removal of Pb(II) ions were more than 91%, indicated that dobera leaves (DL) is very effective adsorbent for removal of Pb(II) ions from drinking water.
4. Conclusion
The present study showed that dobera leaves (DL) could be used as an effective adsorbent for the removal of lead ions from water. Lead adsorption was found to be pH-dependent and maximum removal was observed at pH 5.0. It was found that the equilibrium data was fitted very well with Langmuir-II equation with maximum capacity as 83 mg/g. Also, the equilibrium data can be modeled by Freundlich isotherm model. The adsorption
![]()
Figure 12. The Dubinin-Radushkevich (D-R) the adsorption of Pb(II) onto dobera leaves (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
![]()
Figure 13. Adsorption of Pb(II) from drinking water (T = 298 K, time = 180 min, [Pb(II)]0 = 30 mg/L, pHi = 5, V = 0.025 L, DL dosage = 1.0 g/L).
of Pb(II) was favored by dobera leaves with value of RL less than unity. Furthermore, the adsorption process is spontaneous and follows pseudo-second-order kinetic. In addition the adsorption mechanism involved more than one step. DL have a very good selectivity for Pb(II) in presence of Ni(II) with large ![]() for Pb(II) as 1874.3 mL/g. The present work reveals that dobera leaves are a promising material for the removal of Pb(II) ions from drinking water.
for Pb(II) as 1874.3 mL/g. The present work reveals that dobera leaves are a promising material for the removal of Pb(II) ions from drinking water.
Acknowledgements
The Authors sincerely appreciate financial support from Deanship of Scientific Research, Jazan University, Jazan, KSA, for (project number 004/1431). Also, Authors appreciated valuable discussion from Prof. Dr. Hassan Shehata, Al-Azhar University, Cairo, Egypt.
NOTES
*Corresponding author.