1. Introduction
Increasing prevalence of multiresistant bacteria, mainly related to hospital environment, is a public health problem and elicits a negative economic impact due to increasing time of hospitalization, which brings morbidity and high costs associated with increased number of pharmacological approaches trying to counteract multiresistant bacteria and restore health [1] [2] . The inadequate use of antimicrobial and recurrent bacterial infections generates resistant strain [3] . Considering that bacteria becomes resistant in about 10 hours through mutations and that it takes about 10 to 24 years to develop new drugs, research focusing on compounds with promisor antibacterial activity is warranted [4] .
In this context, venom from venomous animals is a rich source of protein and non-protein compounds of pharmacological interest [5] . The antimicrobial activity of the Bothrops jararaca (Taxonomy ID: 8724) crude venom was showed against Staphylococcus aureus [6] and the isolated peptide from B. jararaca (Pep5Bj) inhibited the growth of different fungi (Fusarium oxysporum, Colletotrichum lindemuthianum, Candida albicans and Saccharomyces cerevisiae) [7] . Considering the relevance of the topic, we conducted a study on the antibacterial potential of Bothrops jararaca crude venom against bacterial clinical isolates.
2. Material and Methods
2.1. Chemicals
Bothrops jararaca crude venom was gently supplied by Fundação Estadual de Produção e Pesquisa em Saúde- Centro de Informação Toxicológica do Rio Grande do Sul (FEPPS/CIT-RS) (Porto Alegre, RS, Brazil). Albumin bovine serum was purchased from Sigma-Aldrich (St. Louis, MO, USA). Culture medium (Müeller-Hinton agar, blood agar, and Müeller-Hinton broth) were purchased from Oxoid Limited (Brooklin Novo, SP, Brazil). Positive controls (imipenem, polymyxin B, and vancomycin were purchased from Diagnósticos Microbiológicos Especializados (Araçatuba, SP, Brazil) and McFarland scale was obtained from Probac Brasil Produtos Bacteriológicos Ltda (Santa Cecília, SP, Brazil).
2.2. Protein Quantification of B. jararaca Venom
Crude venom was lyophilized in order to prevent proteolysis [8] and was kept at −20˚C. Protein content was quantified through Bradford method using albumin bovine serum as standard [9] . Lyophilized venom was suspended in NaCl 0.9%, under laminar flow, and filtrated using a 0.20 µm membrane (Sartorius Stedim Biotech, Göttingen, LS, Germany) in order to obtain a concentrated solution containing 2 mg/mL of protein. New solutions were prepared daily.
2.3. Evaluation of Antibacterial Activity through Agar Diffusion Method
Gram-negative bacteria strains (Acinetobacter baumannii, Oxacillinase-producing Acinetobacter baummanii, extended-spectrum β-lactamase-producing (ESBL) Escherichia coli, ESBL-producing Escherichia coli, Klebsiella pneumoniae, ESBL-producing Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis and metallo β-lactamase-producing Pseudomonas aeruginosa) and Gram-positive bacteria strains (Staphylococcus aureus, oxacillin resistant Staphylococus aureus (ORSA), Staphylococcus epidermidis, and oxacillin resistant Staphylococus epidermidis) were used in the present study. All these bacteria were clinical isolates from Laboratório de Análises Clínicas do Hospital São Vicente de Paulo collection. Moreover, ATCC and NEWP bacteria were also used: Enterobacter aerogenes (NEWP 0048), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (NEWP 0083), Proteus mirabilis (NEWP 0133) and Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (NEWP 0038) and Staphylococcus epidermidis (NEWP 0128).
In order to allow bacterial growing, clinical isolates were kept in blood agar at 37˚C, for 24 hours, in aerobic conditions. ATCC and NEWP strains were recovered in nutritive broth and cultured in Petri dishes containing Müeller-Hinton agar. After growing, bacterial colonies were collected and suspended in 5 mL de NaCl 0.9%. Turbidity was adjusted to 0.5 in McFarland scale, which corresponds to 2 × 108 UFC/mL [1] . Antibacterial activity assay was performed after 24 hour after the turbidity adjust.
Diffusion disks containing 10 µL of crude snake venom solutions in a concentration range of 2 mg/mL, 500 µg/mL, 250 µg/mL, 100 µg/mL, 50 µg/mL, and 25 µg/mL was added to petri dishes with above cited bacteria strains. Negative control was NaCl 0.9% and positive controls were disks containing imipenem (10 µg/mL), vancomycin (30 µg/mL), and polymyxin B (300 µg/mL) to enterobacteria, Pseudomonas aeruginosa and Staphylococcus aureus, respectively. Dishes were incubated at 37˚C, for 24 hours, as recommended by Clinical Laboratory Standards Institute [10] . Antibacterial activity was considered in the presence of an inhibition zone around the diffusion disk, measured in millimeters. Each condition was tested in triplicate.
2.4. Evaluation of Minimum Inhibitory Concentration (MIC) through Microdilution Method
The MIC was determined for the most promisor clinical isolates using serial dilutions (1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024, 1:2048) of crude snake venom (initial concentration of 2 mg/mL) [11] . Five μL of bacterial inoculum was added (0.5 of McFarland scale) and plates were incubated for 24 hours at 37˚C. After this incubation period, plates were revealed with 10 µL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl- tetrazolium bromide (MTT). The NaCl 0.9%, Müeller-Hinton broth, snake venom at 2 mg/mL alone, and culture medium containing bacterial strains were used as sterility controls. Imipinem, polymyxin B, and vancomycin were used as positive controls, as already described above. This assay was performed in triplicate.
2.5. Statistical Analysis
Statistical analysis was performed using One-Way ANOVA followed by Tukey post hoc test. Results presenting a p < 0.05 were considered as statistically significant.
3. Results
Broad Antibacterial Activity of Crude B. jararaca Venom
The protein content of the crude venom was 245 µg/mL. The present work evidenced that crude B. jararaca venom presented antibacterial activity against all clinical isolates, ATCC and NEWP bacterial strains tested, as shown in the Table 1 (images with the agar diffusion test are shown in supplementary material).
The magnitude of inhibition zones showed that better results were obtained against Gram-positive bacteria, especially oxacillin resistant Staphylococcus aureus. Hence, MIC assay was performed only for Gram-positive bacteria (Table 2).
4. Discussion
The major finding of this work was to evidence the wide spectrum antibacterial activity of crude venom isolated from B. jararaca. For the first time, it was evidenced that crude snake venom was effective against both Gram- positive and Gram-negative bacteria from commercial sources and clinical isolates presenting resistance mechanisms. It highlights that it might be considered a promisor source to be explored by pharmaceutical industry.
Snake venoms are a rich source of bioactive compounds to different pharmacological activities due to their complex composition. About 90% to 95% wet weight of the venom corresponds to proteins [12] [13] . Many proteins isolated from snake venoms present enzymatic activity, such as colinesterases, aminotransferases, ATPases, β-glucosaminidases, catalases, phosphodiesterases, phospholipases A2, hyaluronidase, L-amino acid oxidase (LAAO), metalloproteinases, NAD nucleosidases, proteases, and serineproteases [8] [13] [14] .
To the best our knowledge, it is the first time that crude venom from B. jararaca presents wide spectrum antibacterial activity, being effective against all Gram-positive and Gram-negative tested strains, even those expressing resistance mechanisms. In the past, venom from B. jararaca (0.8 mg/mL) inhibited bacterial growth of Eubacterium lentum, Peptoestreptococcus anaerobius, Porphyromonas gingivalis, Prevotella intermedia, Propionibacterium acnes, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus and Staphylococcus epidermidis. However, it failed against Bacteroides fragilis, Eikenella corrondes, Enterococcus faecalis, Escherichia coli and Streptococcus mutans [5] . Moreover, Ferreira (2007), using the high concentration of 5 mg/mL demonstrated that B. jararaca venom presents antibacterial activity only against Staphylococcus aureus. However, in that study, author used a conservative criterion since it was considered positive for antibacterial activity inhibition zones ≥ 15 mm. It is important to point that other bacteria also presented inhibition zones, namely Enterobacter cloacae (6 mm), Enterococcus faecalis (13 mm), Escherichia coli (5 mm), Pseudomonas
![]()
Table 1. Inhibition zones around diffusion disks containing antibiotic (positive control) or B. jararaca crude venom, indicating antibacterial activity. Results are expressed in mm of diameter (mean of 3 independent experiments).
(Control+) Imipenem (enterobacteria), polymyxin B (Pseudomonas aeruginosa), and vancomycin (Staphylococcus aureus). (-) Absence of inhibition zone. a,b,c,d,e,fMeans followed by different letters differ from each other (p < 0.05).
![]()
Table 2. MIC from crude venom of B. jararaca against Gram-positive clinical isolates.
aeruginosa (8 mm), Proteus mirabilis (7 mm), Staphylococcus epidermidis (10 mm), Serratia marcencens (7 mm) and Klebsiella pneumoniae (9 mm). Even so, it was not observed any inhibition zone against Acinetobacter calcoaceticus [15] .
It is important to refer that the present study is a screening since crude venom was used. Regarding promising results obtained, next steps involve working with different isolated fractions from B. jararaca in order to elucidate which fraction are responsible/more effective to the antibacterial activity. Considering known compounds present in the bothropic venom, the enzyme LAAO is frequently associated to antibacterial activity [16] . LAAO is an oxidoreductase involved in the stereospecific oxidative desamination of L-amino acids, producing ammonia and hydrogen peroxide which can cross membranes and induce the oxidation of macromolecules such as protein, lipids and DNA [5] [16] -[19] .
5. Conclusion
In summary, the present work evidenced that B. jararaca venom is a promising source from antibiotics development research. Its antibacterial activity is warranted to be explored considering the good results even with clinical isolates presenting resistant mechanisms. However, additional studies are needed in order to elucidate which substance in this complex matrix is in charge of antibacterial activity.
Acknowledgements
Authors thank to Centro de Informações Toxicológicas do Rio Grande do Sul for kindly provide Bothrops jararaca venom and to Laboratório de Análises Clínicas from Hospital São Vicente de Paulo de for the bacteria.
Supplementary Material
Inhibition zones after around diffusion disks containing antibiotic (positive control) or B. jararaca crude venom, indicating antibacterial activity.
Acinetobacter baumannii
C+: Positive control (Imipinem) = 35 mm.
MS: Mother solution of B. jararaca venom (2 mg/mL) = 10 mm
1: B. jararaca venom 500 µg/mL = 9 mm
2: B. jararaca venom 250 µg/mL = 8 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
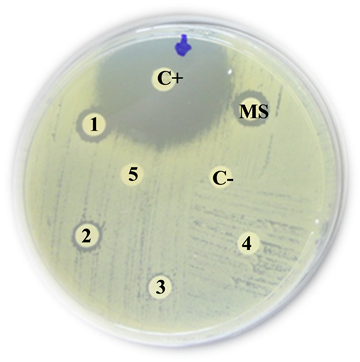
Oxacillinase-producing Acinetobacter baummanii
C+: Positive control (Imipinem) = 10 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 11 mm
1: B. jararaca venom 500 µg/mL = 10 mm
2: B. jararaca venom 250 µg/mL = 9 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL =0 mm
C−: Negative control (saline solution) = 0 mm
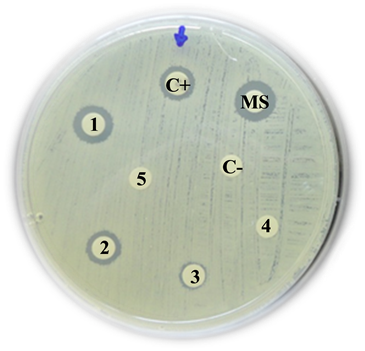
ESBL-producing Enterobacter aerogenes
C+: Positive control (Imipinem) = 27 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 10 mm
1: B. jararaca venom 500 µg/mL = 9 mm
2: B. jararaca venom 250 µg/mL = 8 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
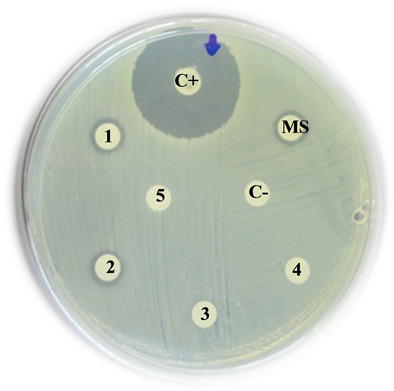
Escherichia coli
C+: Positive control (Imipinem) = 35 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 9 mm
1: B. jararaca venom 500 µg/mL = 8 mm
2: B. jararaca venom 250 µg/mL = 0 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
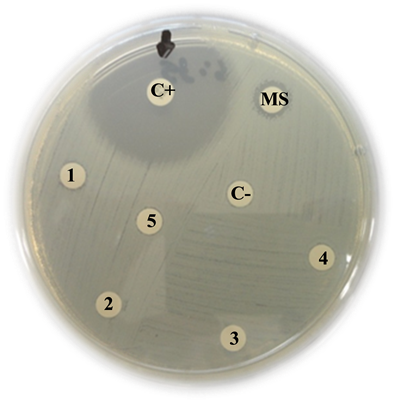
ESBL-producing Escherichia coli
C+: Positive control (Imipinem) = 36 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 10 mm
1: B. jararaca venom 500 µg/mL = 9 mm
2: B. jararaca venom 250 µg/mL = 7 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm

Pseudomonas aeruginosa
C+: Positive control (Polymyxin B) = 15 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 11 mm
1: B. jararaca venom 500 µg/mL = 9 mm
2: B. jararaca venom 250 µg/mL = 8 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
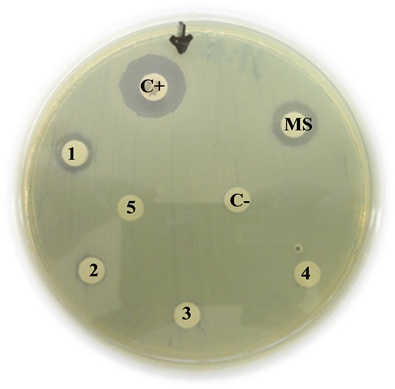
metallo β-lactamase-producing Pseudomonas aeruginosa
C+: Positive control (Polymyxin B) = 15 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 10 mm
1: B. jararaca venom 500 µg/mL = 10 mm
2: B. jararaca venom 250 µg/mL = 7 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm

Klebsiella pneumoniae
C+: Positive control (Imipinem) = 36 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 10 mm
1: B. jararaca venom 500 µg/mL = 8 mm
2: B. jararaca venom 250 µg/mL = 0 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm

ESBL-producing Klebsiella pneumoniae
C+: Positive control (Imipinem) = 29 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 10 mm
1: B. jararaca venom 500 µg/mL = 8 mm
2: B. jararaca venom 250 µg/mL = 0 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
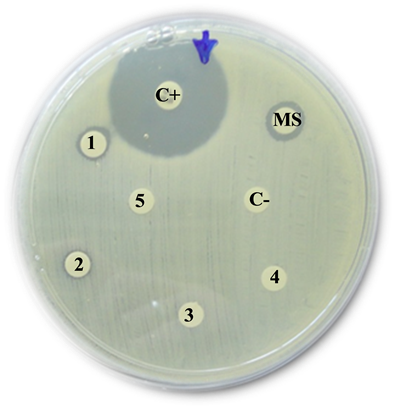
Enterococcus faecalis
C+: Positive control (Imipinem) = 23 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 10 mm
1: B. jararaca venom 500 µg/mL = 0 mm
2: B. jararaca venom 250 µg/mL = 0 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
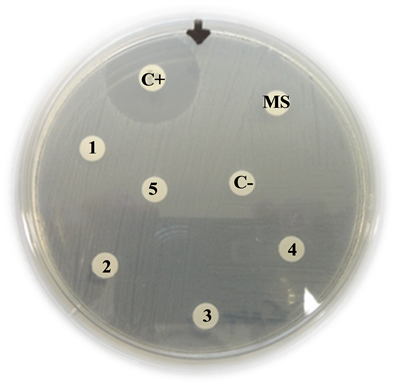
Proteus mirabilis
C+: Positive control (Imipinem) = 13 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 12 mm
1: B. jararaca venom 500 µg/mL = 11 mm
2: B. jararaca venom 250 µg/mL = 9 mm
3: B. jararaca venom 100 µg/mL = 0 mm
4: B. jararaca venom 50 µg/mL = 0 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
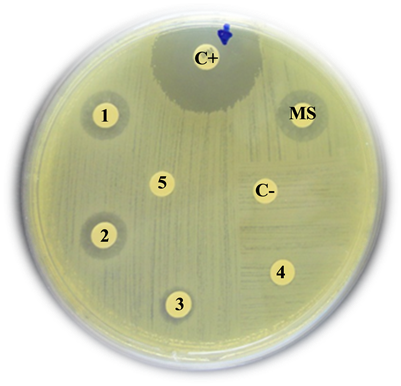
Staphylococcus aureus
C+: Positive control (Imipinem) = 20 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 16 mm
1: B. jararaca venom 500 µg/mL = 14 mm
2: B. jararaca venom 250 µg/mL = 11 mm
3: B. jararaca venom 100 µg/mL = 10 mm
4: B. jararaca venom 50 µg/mL = 8 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
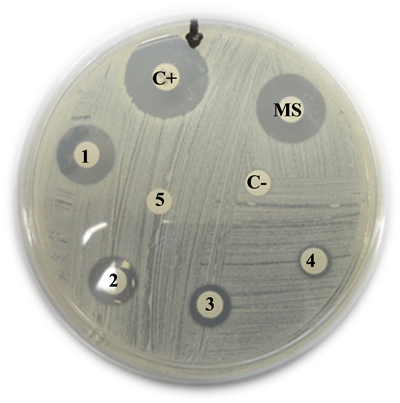
Staphylococus aureus
C+: Positive control (Vancomycin) = 21 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 16 mm
1: B. jararaca venom 500 µg/mL = 15 mm
2: B. jararaca venom 250 µg/mL = 14 mm
3: B. jararaca venom 100 µg/mL = 10 mm
4: B. jararaca venom 50 µg/mL = 9 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
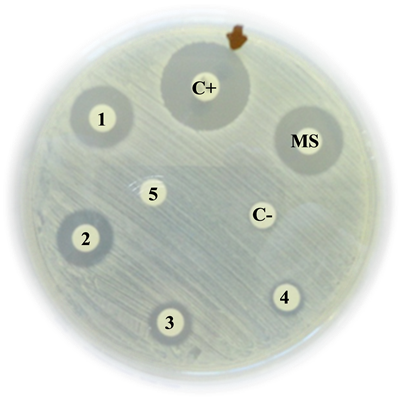
Staphylococcus epidermidis
C+: Positive control (Vancomycin) = 42 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 13 mm
1: B. jararaca venom 500 µg/mL = 12 mm
2: B. jararaca venom 250 µg/mL = 11 mm
3: B. jararaca venom 100 µg/mL = 10 mm
4: B. jararaca venom 50 µg/mL = 9 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
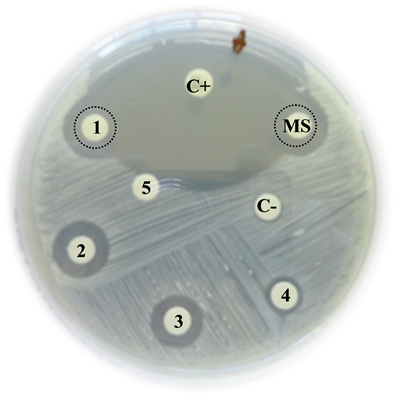
OSRA
C+: Positive control (Imipinem) = 25 mm
MS: Mother solution of B. jararaca venom (2 mg/mL) = 15 mm
1: B. jararaca venom 500 µg/mL = 14 mm
2: B. jararaca venom 250 µg/mL = 13 mm
3: B. jararaca venom 100 µg/mL = 10 mm
4: B. jararaca venom 50 µg/mL = 9 mm
5: B. jararaca venom 25 µg/mL = 0 mm
C−: Negative control (saline solution) = 0 mm
![]()
NOTES
*Corresponding author.