1. Introduction
The so-called polyols or polyhydroxy alcohols are reduced forms of sugars which are probably ubiquitous in all plant species [1] . The most common polyols are derived from hexose sugars through the reduction of aldose or ketose, which are reduced to a hydroxyl group by aldose reductase (alditol/NADP+ oxidoreductase, E.C.1.1.1.21, ALR2), considering it the first enzyme in the polyol pathway which reduces D-glucose to D-sorbitol, and with the concomitant conversion of NADPH to NADP [2] . Therefore, mannitol, sorbitol (or glucitol) and dulcitol (or galactitol) are the equivalent polyols of hexose glucose, fructose and galactose, respectively, and are hexitols frequently found in angiosperms [3] .
As mannitol is the most abundant polyol in nature, it participates in the translocation and storage of metabolites, in addition to providing resistance to salinity and osmotic stress in plants [3] , and it can also act as protection against invading pathogens [1] . D-sorbitol is a primary product of photosynthesis and studies have shown that it is very common, especially in fruit [1] . It is very abundant in some species, such as in apple and also in mulberry. D-sorbitol has approximately the same levels as sucrose [4] .
Despite the importance, occurrence, metabolism and physiology of polyols in plants, studies are needed for this type of compound as its function in plants is not completely known. The absence of a chromophore cluster (benzene ring) in the molecule of D-sorbitol results in the disadvantage of high performance liquid chromatography analysis (HPLC), as it precludes its detection by the absorbance of non-derivatized sugar at wavelengths above 200 nm.
The non-derivatized sugars can usually be analyzed and quantified using a variety of chromatographic methods in conjunction with different types of detectors such as: refraction rate, electrochemical detection and currently by evaporative light scattering detection. Absorbance and fluorescence detection has advantages such as: low cost, derivatization feasibility in pre and post-column stages of carbohydrates, increased sensitivity and selectivity detection [5] .
The pre-column derivatization of sugars using compounds that have strong UV-adsorbent chromophore facilitates monitoring absorbance at the 230 to 280 nm region. The benzylation process covers a large class of substances containing hydroxyl, amino groups and other compounds, which in the presence of water results in UV-active derivatives that are suitable for extraction in organic solvents [2] . The benzene radical easily connects to hydroxyl groups of several molecules, also including the sugars and polyhydroxy alcohols. Since these groups develop double bonds, the derivatives containing the benzene radical absorb in the UV-visible molecules of any source. Thus they can be detected by a UV type detector [6] .
Considering the difficulties in the microanalysis method (detection and quantification) of sugars and polyalcohols (mannitol and sorbitol), as well as the costly liquid-liquid extraction technique used in gas chromatography (GC), this study describes a methodology for the analysis of D-sorbitol using benzyl alcohol as derivatizing agent and liquid chromatography equipped with a diode array detector (HPLC-DAD).
2. Materials and Methods
The study was carried out at the Sanitation Laboratory of the University of São Paulo State (UNESP), Ilha Solteira Campus, Brazil. Axillary buds from shoots were collected at UNESP Teaching and Research Farm, the fruit trees were: avocado (Persea americana Mill); black mulberry (Rubus brasiliensis); Japanese pear (Pyrus pyrifolia var culta) and peach (Prunus persica), guava (Psidium guajava L.) and mango (Mangifera indica L.).
2.1. Sample Preparation
The axillary buds of fully developed leaves located in the branches, in that period of growth, were collected in 10/09/2009. The shoots were kept separate in methanol (MeOH 80%) and under refrigeration prior to the benzylation steps and solid phase extraction of sorbitol.
The bud samples were dried in an oven at 65˚C for 24 hours, to later determine the mass of each sample using a 5 digit analytical balance. These samples were then macerated in a 10.0 mL Hach tube using a polyethylene stick. 2.0 mL of MeOH (100%) were used for the liquid phase extraction of sorbitol. The samples were then homogenized for 15 min using a vortex (BiomiXer). The macerated sediment was filtered using a qualitative filter paper and then underwent the derivatization step.
2.2. Derivatization
The methods proposed in [7] and [6] were used in the benzylation step (pre-column derivatization). The benzylation was performed with 50.0 mL of benzyl alcohol concentrated in the fruit tree bud samples, and homogenization was performed by vigorous shaking in the vortex for 1.0 min; 50.0 mL of benzyl alcohol concentrate was again used to facilitate the benzylation reaction of the other compounds that could be present in all samples; a new homogenization was carried out for 1.0 to 2.0 min and then 100.0 mL of sodium hydroxide (8 M) was added and all samples were homogenized for 5.0 min. Using a digester (Hach-DRB 200), the samples were left to
rest for 20 min at 30˚C, then neutralization (basifying) of the samples was performed with 100.0 mL of phosphoric acid (1.4 M) and then homogenization was carried out for 1.0 min.
The benzylated sorbitol in the fruit tree bud samples was subjected to solid phase extraction (C18 cartridges) [6] . The C18 cartridges were initially pre-activated with 10.0 ml of MeOH (100%) and 10.0 mL of Milli-Q water at a flow rate of 3.0 mL∙min−1; the derivatized samples were then eluted at a speed of 3.0 mL∙min−1; next, 2.0 mL of Milli-Q water was added and the eluate was collected by the addition of 500 mL of acetonitrile (60%) and then again the addition of 500 mL of acetonitrile (100%).
2.3. Validation of the Method
For the method validation a spread sheet proposed in [8] was used to analyze the following parameters: selectivity, adjustment of the analytic curve and determination of its linearity range, sensitivity of the method, repre- sented by the limits of detection (LD) and quantification (LQ), precision, accuracy and robustness. The recovery (which evaluates the efficiency of the method) of sorbitol was carried out through three aliquots (0.04; 0.070 and 0.1 µg∙mL−1) resulting from the analytical curve of the D-sorbitol samples (HPLC grade) and benzyl alcohol/ NaOH (basification), whose samples were subjected to solid phase extraction using C18 cartridges as described earlier. The samples were injected into the HPLC five times [9] .
A way to ensure the applicability and scope of a method for the routine operations of a laboratory is establishing the limits of parameters from the estimation of figures of merit, known as a validation step [8] .
Quantitative indicators of the scope and the good performance of the techniques, and are described in the literature as: selectivity, adjust the calibration curve and determination of its linearity range, sensitivity of the method represented by the limits of detection (LD) and quantification (LQ), precision, accuracy and robustness [10] - [12] .
Selectivity is the ability to assess unequivocally the substances under examination in the presence of components that may interfere with their determination in a complex sample [9] . This ensures that the signal is not affected by interfering substances.
Linearity expresses the range in which the analytical signal, called the dependent variable yi is linearly proportional to its concentration, called the independent variable xi, and the mathematical equation that describes this dependence is known as calibration curve or calibration curve [8] .
Adjusting any mathematical equation is done by the least squares method, in which the best curve will be one that will provide the lowest value for the sum of the quadratic residues (Q) obtained between the measured analytical signal (yi) and the predicted analytical signal (ŷi) for a set N data points. The curve fit using the method of least squares regression coefficients provide, in the case of a linear fit (yi = a0 + a1xi) is linear (yi = a0 + a1xi) and
angular coefficients (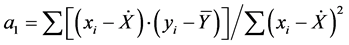 ), where x is the mean value of the concentration
), where x is the mean value of the concentration
(xi) to the total number of samples (N) used as calibration standards.
The sensitivity of a method is defined by the limits of detection (LD) and quantification (LQ). The LD is the lowest concentration of the species of interest that can be detected by instrumental technique, while the LQ is the lowest concentration that can be quantified within the limits of precision and accuracy of the method during routine operation of the laboratory, under usual conditions [8] .
The LD and LQ can be estimated based on the parameters of the analytical curve method [8] . The method of estimation of LD based on parameters of the analytical curve shows greater statistical reliability because it takes into account the confidence interval of the regression [8] . LQ can be determined by analysis of samples with known concentrations of the species of interest and corresponds to the lowest concentration that can be quantitated within the limits of accuracy and precision of the method [9] . The LQ is calculated from the confidence interval of the analytical curve.
The term provides precisely the dispersion of the measured values around a mean value, and its numerical value is estimated by the relative standard deviation, RSD or (“relative standard deviation”) for analysis of samples containing the same amount of the species of interest [8] .
The accuracy can be estimated at three levels: repeatability, intermediate precision and reproducibility [8] . The term repeatability, accuracy or within-run precision, defines the accuracy of the results obtained within the laboratory itself, by the same analyst with the same instrumentation. The intermediate precision, accuracy or iter-run defines the precision within a laboratory for measurements obtained by different analysts, or on different days or for different equipment. The term reproducibility defines the accuracy of the results obtained for a given analysis performed by different laboratories, but following the same methodology. The precision reflects the closeness of the measured value (xi) and a reference value considered true (xv) and is correlated with the absolute error of a measured [8] .
The robustness of a method is its ability to withstand small variations of the analytical parameters without significantly altering its accuracy and precision, thus is a measure of the amount of variability that the method can support without losing reliability, and its estimate depends on the type of analytical methodology. The robustness of a method may be estimated by varying the analytical parameters and comparing the precision obtained in each determination [8] .
2.4. Chromatographic Conditions
The chromatographic analysis was performed in two phases, the first step was qualitative and the second quantitative. In the first stage the objective was to detect sorbitol in the bud and in the second step the quantification of sorbitol in the species in which its presence was detected.
Chromatography was performed using a Shimadzu HPLC system equipped with an LC column chromatography Column Shim-pack C18 (250 mm × 4.6 mm ID, 5.0 mm particles) with a guard Column Shim-pack C18 (50 mm × 4.6 mm ID, 5.0 mm particles). Elution was carried out using acetonitrile-water (gradient of 20% to 80% of acetonitrile for 30 min). The detection of the chromatographic peaks was performed by a SPD-M20A detector (prominence Diode Array detector) using wavelengths at 228 nm and 248 nm. The chromatograms were integrated using the LC solution software.
3. Results and Discussion
The C18 LC Column Shim-pack column showed excellent chromatographic resolution and a clear separation of D-sorbitol (derivatized) with different degrees of purity (Figure 1(a)) and the axillary buds of fruit trees such as: avocado, black mulberry, Japanese pear and peach (Figure 1(b)) respectively.
Table 1 shows the parameters values of: sensitivity (LD and LQ), precision and accuracy performed by the analytical curve of the derivatized D-sorbitol and recovery (SPE). According to the Student’s T test (tcalculated), there are no significant differences between the recoveries and the expected value (100%), since all the absolute values of tcalculated are smaller than the value tabulated for t, therefore it was found that the method used for the microanalysis of derivatized D-sorbitol can be considered precise and accurate.
The intervals for the coefficients of variation (1.4 to 8.6) resulting from the recovery of D-sorbitol proved to be suitable, since they do not exceed the limits (20%) [8] .
Thus, the results in Table 1 regarding the accuracy and precision requirements for the fortifications levels studied on the same day, with the same analyst for the method used; with the same instrument and at the same location, with repetitions at short time intervals, verifies the efficiency for the method used to quantify the derivatized D-sorbitol.
![]()
Table 1. Parameter values of: limits of detection (LD) and quantitation (LQ), precision and accuracy for the derivatization method of D-sorbitol conducted by a validation spreadsheet proposed in [8] .
R2: correlation coefficient; Χ: average of quintuplicates; σ: standard deviation; C.V.: coefficient of variation.
![]()
Figure 1. (a) Spectrophotometric and chromatographic profiles of derivatized D-sorbitol. (1) Benzyl alcohol (T.r. 5.24); (2) derivatized D-sorbitol HPLC grade (T.r. 7.27) and (3) commercial use derivatized D-sorbitol (T.r. 7.27). LC column shim-pack C18 ( 250 mm × 4.6 mm ID, 5.0 mm particles), detection at 228 nm; (b) Chromatographic profiles of derivatized D-sorbitol found in the buds of different fruit species. (1) Avocado, (2) black mulberry, (3) Japanese pear, (4) peach, (5) derivatized D-sorbitol (5.0 mm particles). LC column shim-pack C18 ( 250 mm × 4.6 mm ID, 5.0 mm particles), detection at 228 nm.
The derivatives of D-sorbitol-benzyl absorbing ultraviolet light resulting from the reaction between the D- sorbitol (HPLC grade) and benzyl alcohol/NaOH (basification) and also the macerated axillary buds of fruit trees showed stability for several hours (24 to 48 hours), allowing them to be detected and quantified by HPLC. In the present study the use of benzyl alcohol as reagent of benzylation for the microanalysis of polyols (sorbitol) are also in agreement with [7] and [13] with respect to the stabilization time of the derivatization reaction. These authors also observed a reduced stabilization time in the reaction of benzoyl chloride with D-sorbitol in sugar, polyols and amino acids from human biological fluid samples.
Table 2 shows the levels of D-sorbitol (benzylated) of interest in the samples of axillary buds of fruit trees. The fructose, glucose and lactose contents are well known in fruits and vegetables [14] , however the levels of sugar polyols (sorbitol and mannitol) are limited to only some literature data.
Studies show that the levels of D-sorbitol may vary between different species or even among the same gender during their maturation stage. Recent studies on different types of fruit have demonstrated a significant difference in the levels of D-sorbitol (grams/100 g of dried fruit) in: blackberry (4.76 g), avocado (0.65 g), lychee (0.53 g), longan (0.68 g), nectarines (1.01 g) and depending on the species and type of processing in apples (0.14 to 0.73 g), pear (0.90 to 5.99 g) and peach (0.90 to 5.99 g) [14] .
In this study the levels of D-sorbitol resulting from the averages of three concentrations found in the axillary
![]()
Table 2. Derivatized D-sorbitol levels (µg∙mg−1 dry wt) found in axillary buds of different fruit trees.
n.d.: not detected; Χ: average of triplicates; σ: standard deviation; C.V.: coefficient of variation.
buds of fruit trees were limited to the order of µg∙mg−1 dry wt (Table 2), obtaining the levels of D-sorbitol in larger axillary buds for Pyrus pyrifolia var culta (0.138 µg∙mg−1) followed in descending order by Persea americana Mill (0.099 µg∙mg−1), Rubus brasiliensis (0.042 µg∙mg−1) and Prunus persica (0.040 µg∙mg−1). The literature shows that Rubus sp. and Pyrus sp. are the species with the highest levels of D-sorbitol. However, this was not verified for the levels of D-sorbitol in the axillary buds of Rubus brasiliensis (Table 2).
The low activity or the enzyme deficiency of sorbitol dehydrogenase could be a possible explanation for the low D-sorbitol content found in the axillary buds of Rubus brasiliensis and even for Prunus persica [14] . Because it is known that the enzyme NAD-SDH found in fruits (apricot, loquat, apple, peach, pear) and other higher plants (squash, broccoli, fennel, beans, cucumber) [14] is responsible for the oxidation of glucose for sorbitol and fructose [1] . In addition to being considered the key to the metabolic utilization of D-sorbitol, it also plays an important role in carbon supplementation during the development of leaves and fruits [15] .
The lack of NAD-SDH or even the lack of activity of this enzyme in many young leaves and also its detection difficulty in mature leaves suggests an interrelationship between the development of the chloroplast, photosynthetic capacity and in turn in the synthesis of D-sorbitol [2] . Studies using experiments with pulse markers (14CO2) in fully expanded pear leaves demonstrated that D-sorbitol was poorly metabolized and suggested that the regions of meristematic growth activity (such as the axillary buds) were probably using the D-sorbitol, therefore the maturation of plant tissues tend to lose this ability. This report might corroborate the low content of D-sorbitol found in the axillary buds of Rubus braziliensis, which could probably be due to the low activity or even the lack of aldose reduction.
The variation between the D-sorbitol levels can be expected, as previously mentioned, for the different fruit species or even depending on the variations occurring among their own gender, the season, weather, storage time and temperature [16] . Therefore, considering the differences in: maturation, cultivation and geographical location, the results can vary greatly regarding the contents of this polyalcohol [6] .
The results demonstrated the occurrence of D-sorbitol in avocado, black mulberry, peach and Japanese pear, which confirms that the polyalcohol is in fact widely dispersed in some fruits, except for: banana, citrus fruits, mango, melon and grapes [4] and [14] .
The proposed derivatized D-sorbitol method enabled eliminating the use of the most hazardous derivatizing agents, as well as reducing the running time, when compared to gas chromatography.
4. Conclusion
The derivatization method proposed for HPLC-DAD showed excellent chromatography resolution and high accuracy in the separation of D-sorbitol (derivatized) for the axillary buds of avocado, black mulberry, Japanese pear, peach, as well as enabled eliminating the use of more dangerous derivatizing agents.
NOTES
*Corresponding author.