Tree Allometry in Tropical Forest of Congo for Carbon Stocks Estimation in Above-Ground Biomass ()
1. Introduction
Tropical forests are a key component of the global carbon cycle (Djomo et al. 2010 and Ekoungoulou et al. 2014a) . While the Congo basin is the second largest block of continuous tropical forest after the Amazonian basin, there is still a lot of uncertainty about the amount and spatial variations in above-ground biomass (biomass hereafter) and carbon stocks (Zhang et al. 2002 and FAO, 2011) . The most voluminous greenhouse gas produced by humans is carbon dioxide (CO2) as mentioned by Fayolle et al. (2013) . In calculating overall carbon emissions, Kyoto Protocol allows certain removals of carbon by a nation’s forests and soils carbon sinks to be counted and deducted from emissions (FAO, 2008) . Thus, one option for mitigating greenhouse gas emissions and thus possible climate change is to increase the amount of carbon stored in forests (Gorte, 2009) . Carbon sequestration, and the extent to which it can be counted as a reduction in a nation’s carbon emissions, have been the focus of substantial controversy in international negotiations subsequent to the Kyoto Protocol ( Gorte, 2009; Fayolle et al. 2013 and Holdaway et al. 2014 ). Global climate change is a widespread and growing concern that has led to extensive international discussions and negotiations (IPCC, 2001) . One response to this concern have focused on reducing emissions of greenhouse gases, especially carbon dioxide (Brown et al. 2004) , and on measuring carbon absorbed by and stored in forests, soils, and oceans. One option for slowing the rise of greenhouse gas concentrations in the atmosphere, and thus possible climate change, is to increase the amount of carbon removed by and stored in forests. Deforestation and forest degradation, located primarily in tropical regions, accounted for 12% - 20% of global anthropogenic greenhouse gas (GHG) emissions in the 1990s and early 2000s (IPCC, 2001) . And these processes also impact the future potential of forests to remove additional carbon from the atmosphere ( Gorte, 2009 and IPCC, 2011 ). Estimates of greenhouse gas emissions from deforestation require information on both the area of forest loss and the corresponding carbon stock of the land that is cleared (Chave et al. 2005 and Alvarez et al. 2012) . Both are considered challenging to quantify accurately (Brown, 2002) . Much of the emphasis to date has focused on improving spatially represented estimates of forest area loss (Brown et al. 2000 and FAO, 2008) . To improve confidence in estimated emissions, equal emphasis is needed on improving spatially explicit estimates of carbon stored in forests, which remain uncertain in tropical regions. The largest proportion of this uncertainty is in estimates of aboveground biomass (Kearsley et al. 2013 and Ekoungoulou et al. 2014a) , which accounts for 70% - 90% of forest biomass carbon (IPCC, 2001 and FAO, 2008) , and its spatial variability that depends on factors such as climate, human and natural disturbance and recovery, soil type, and topographical variations (IPCC, 2011) . Many techniques exist to estimate forest biomass at different spatial scales, but they all ultimately rely on ground and destructive measurements of individual tree biomass to calibrate allometric equations (Hall, 2012) . An allometric equation is a statistical model relating tree biomass to a set of predictors such as tree diameter and/or height, wood specific gravity, or forest type (Chave et al. 2005 and Gorte, 2009) . Allometric equations are used to convert forest inventory data into biomass estimates at tree-level, and the sum of all data for the trees allows a biomass estimate to be obtained at plot level (White et al. 1986; Chave et al. 2001 and Alvarez et al. 2012) . Since existing allometric equations for tropical trees in African moist forests are restricted to a few specific species or sites (IPCC, 2001 and Djomo et al. 2010) , pan- tropical multi-species equations are being used instead of estimating biomass from inventory data (Killeen et al. 2002; Lewis et al. 2009 and IPCC, 2011) . Plot-based estimates of forest carbon stocks and carbon fluxes are derived metrics that contain multiple sources of uncertainty (Phillips et al. 1998; Malhi et al. 2004 and Chave et al. 2008) . Calculations of forest carbon stock are usually based on plot-based field measurements of stem diameter and (occasionally) stem height. The imperfect measurements are transformed into stem biomass estimates, using models introducing model uncertainty (IPCC, 2001) . These include height diameter models to predict tree height and carbon biomass models to predict carbon stock as a function of diameter, height and wood density (Yanai et al. 2010; Saatchi et al. 2011) . Finally, biomass is summed across all stems in the plot and divided by the plot area to give total carbon stock estimated on a per-area basis. This step introduces a second element of measurement error relating to missing or double counted stems and the ability to accurately measure plot area in steep and undulating terrain (Brown et al. 2000 and Brown, 2002) . In this study, we focused on above-ground woody biomass rather than on total above- and below-ground biomass. So, the aim of this study is to estimate the total carbon stock in Iboubikro and Ngambali forests (Lesio-louna area) in Republic of Congo. The results of this research will be useful by the Ministry of Sustainable Development, Forest Economy and Environment of Congo.
2. Materials and Methods
2.1. Study Area
Study area is located at Lesio-louna (14˚E, 4˚S), 140 km North East of Brazzaville in Teke Trays area (Republic of Congo) in Central Africa. Also, Lesio-louna is a wild life reserve that extends over approximately 173.000 ha. Teke Trays area wide range of trays starting from Republic of Gabon crossing Republic of Congo to the Democratic Republic of Congo. However, the average annual rainfall is 2100 mm (2008-2011) with a marked dry season from June to September and an annual average air temperature of 26˚C (ANAC, 2014 and Ekoungoulou et al. 2014b) . The climate of Lesio-louna is a tropical equatorial climate which is characterized by the absence of large dry seasons and low temperature differences (Makany, 1976 and Ekoungoulou et al. 2014a) . Every month, there shall be at least 50 mm of water. There are two rainy seasons (March-May and October-December) and two dry seasons from June to August and from January to February. In Lesio-louna (study area), the average annual rainfall is around 1500 to 2000 mm, but the plates is higher and reaches 2000 to 2500 mm (Makany, 1976 and ANAC, 2014) . Savannas of Teke trays’s South-eastern are wetter than the Southwest, which support only 1400 - 1800 mm. This high rainfall zone extends to Gabon (Anon, 2010) . Inter annual variability is important, however, as is common in regions near the equator (Ekoungoulou et al. 2014b) .
2.2. Data Collection
We used diameter at breast height (DBH) and wood density for each tree in two sites of study area which are Iboubikro site and Ngambali site. Total number of plot in the study area was six (three plots in Iboubikro site and three plots in Ngambali site). Also, six plots are in gallery forest in Lesio-louna protect area. DBH of each tree was measured by using meter tape. The study was done with the circular plots for each site of study area. The size of each circular plot was 1256 m2. About the measurements of this study, the height of the chest was 1.30 m above the ground for each tree using meter tape. This study was done with the use of the compass to know the North, South, East and West positions. Measurements were made from the center of the plot to the North part, then from the center of the plot to the South part as mentioned by Pearson & Brown, (2005) . Following was from the center of plot to the East part, followed from the center of plot to the West part. This work was repeated in all six plots of our study area (Iboubikro and Ngambali sites) in Lesio-louna forest. Thus, we used the GPS to meet the GPS way point of each plot, about a precision of the geographical position of each plot and the entire study area. So, GPS way points were observed in the center of each plot and the compass was used as the center of each plot of this tropical rainforest. However, measuring of diameter at breast height of tree is important that the diameter at breast height’s tape is properly used to ensure the consistency of measurements made. The following steps were respected:
· Make sure you have a bar or a pole with a length of 1.3 m to accurately measure line chest height on the tree (Pearson & Brown, 2005; Ekoungoulou et al. 2014a ). If there is none, using a large piece (2 cm of diameter) in better. In turn, each member of the survey team should measure itself on the location of the breast height is 1.3 m above the ground and rely on this site to determine where to put the meter tape;
· DBH tapes have a hook at the end. Push the hook into the bark of the tree and pull the tape to the right. DBH tape is always from the left and be pulled around the tree, even if the operator who takes action is a southpaw;
· When the tape is worn around the shaft and returns to the hook, the tape should be located above the hook. The tape should be upright and not upside down, the numbers must be in the correct reading order;
· If the tree is on a slope, always measure up the slope;
· If the shaft is tilted, the DBH tape should be worn according to the natural angle of the shaft, without taking into account the slope of the ground relative to the horizontal;
· If the tree has a fork at the chest level, take such action under the fork, and if that is not possible, consider that you are measuring two trees;
· If the tree is lying but still alive, then place the measuring stick down and measure at breast height as if the tree was standing. Trees are considered alive if they have green leaves;
· If a vine is growing on a tree to be measured, we do not cut the vine to clear a space to measure the diameter at breast height. If possible, move away from the vine trunk and drag the tape below. If the vine is too large to be removed from the trunk, we use the back of the ribbon and pull the front of the tree to assess the diameter of first hand. Cutting a vine should be the last option because in the long term with repeated measures taken, interfere with the natural dynamics of the plot eventually differentiate it from the surrounding forest. The same principle has to be respected for any other natural species found on a tree.
2.3. Data Analysis
We developed a set of local site-specific allometric equations. Following Chave et al. (2005) , we fitted the following general allometric model to the observed data:
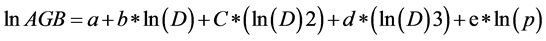
where above-ground biomass (AGB), D and ρ are tree above-ground biomass (kg), diameter (in cm) and wood specific gravity (g∙cm−3), respectively, and a, b, c, d, and e, the model parameters. Three alternative models were derived from model 1. Model 2 was similar to model 1 but assumed that e = 1; model 3 assumed that c = d = 0 and e = 1, and model 4 assumed that c = d = e = 0.
We then estimated the above-ground biomass (AGBest, in kg) of each individual tree from the diameter (D, in cm) and wood specific gravity (ρ in g∙cm−3) using the tropical equation developed by Chave et al. (2005) for moist forests (corresponding to forests with a marked dry season and between 1500 and 3500 mm annual rainfall), as follows:
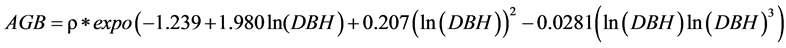
For unidentified species, we applied the mean wood density for each plot weighted, by the number of trees from each species (Ekoungoulou et al. 2014b) .
Then, by Chave et al. (2005) , for the rainforests, the general equation was chosen:
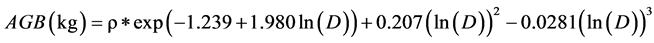
So, if the DBH one tree is 40.3 cm. and his density is ρ =0.05 g∙cm−3. Then, 40.3 cm is well within the maximum diameter at breast height for this equation, which is reliable up to 148 cm as reported by Pearson & Brown, (2005) .
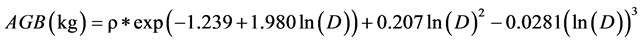
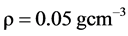
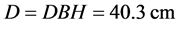
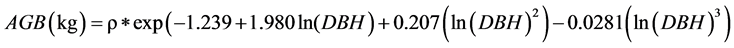
The biomass of this tree is 211.5 kg, or 0.2115 t. Then, to determine the carbon quantity of this tree, we divided the biomass obtained by two (Chave et al. 2005; Pearson & Brown, 2005 and Ekoungoulou et al. 2014b ). So, the carbon stock estimation of this tree is 0.1057 t C.
3. Results and Discussion
In this research, two sites were studied with six plots (three plot each) in Republic of Congo. All six plots are in the gallery forest, precisely in Iboubikro forest and Ngambali forest. Figure 1 shows that, the average of carbon stock about above-ground biomass (AGB) was 204.693 (t C ha−1) for Iboubikro forest and 136.6523333 (t C ha−1) for Ngambali forest. In this forest, the high carbon stock was obtained in Plot 3, followed by plot 1, then plot 2 (Figure 2). The small quantity of carbon was stored in the Plot 4. Thus, there are three plots of Iboubikro forest, including 52 trees measured during the study and three plots of Ngambali forest, including 41 trees. The total number of trees measured in this study for the study sites was 93 trees (Figure 3). The 93 trees in study area are the trees with DBH ≥ 10 cm as mentioned by Fayolle et al. (2013) . However, for the distribution of trees by diameter class, we recorded in Iboubikro and Ngambali forests: 51 trees for the diameter class 10 - 30 cm, 36 trees for the diameter class 30 - 60 cm and 6 trees for the diameter class >60 cm. We obtained the high number of trees in the diameter class 10 - 30 cm which was precisely in Plot 3 with 16 trees recorded. Total number of tree
![]()
Figure 1. Average of carbon stock of above-ground biomass in Ngambali forest and Iboubikro forest (t C ha−1).
![]()
Figure 2. Carbon stock of aboveground biomass in six plots of study area (t C ha−1).
![]()
Figure 3. Total number of trees recorded in six plots studied (Iboubikro and Ngambali).
in Plot 3 was 20 (Table 1). The average of carbon stock about above-ground biomass (AGB) in the study area was 170.6726667 (t C ha−1).
The study was conducted in six plots, which are divided into two study sites (Iboubikro and Ngambali). Iboubikro and Ngambali forests are the galleries forests in Lesio-louna protect area of Congo. However, our study area is fed by two streams (Lesio and Ngambali). Iboubikro site is crossed by the Lesio stream that flows into the Louna stream. On the other hand, Ngambali stream running through our study Ngambali site to throw in the Blue Lake. Blue Lake empties into Louna stream. Thereafter, the stream Louna receives Lesio and Blue Lake’s waters, flows into the Lefini River, then Lefini River flows into the Congo River and the Congo River flows into the Atlantic Ocean. About distribution of trees by diameter class (six plots recorded), we observed in this forest studied: 51 trees for the diameter class 10 - 30 cm, 36 trees for the diameter class 30 - 60 cm and 6 trees for the diameter class >60 cm (Table 1). We obtained the high number of trees in the diameter class 10 - 30 cm which was precisely in Plot 3 (20 trees). Table 1 show that the diameter class 10 - 30 cm has a high number of trees which was 51 trees, followed by diameter class 30 - 60 cm with 36 trees and diameter class >60 cm with 6 trees. In six plots of the study area (Iboubikro and Ngambali forests), the Plot 3 has the highest number of trees (20 trees), followed by Plot 5 (18 trees) and Plot 1 with 17 trees. This gallery forest (Iboubikro and Ngambali sites) has 93 species recorded during this study from August to October 2012. Six plots studied contain a total of 93 trees, including 52 trees recorded in the Iboubikro site and 41 trees recorded in Ngambali site (Figure 3). In 6 plots studied, the large number of trees was recorded in Plot 3 (20 trees), followed by Plot 5 (18 trees), then the Plot 1 (17 trees). A small number of trees have been recorded in Plot 4 (11 trees). All six plots were in circular plots that the size was 1256 m2 for each (Table 2). Each plot consists of three circular radiuses (radius of 6 m for trees 10 - 30 cm DBH; radius of 14 m for trees 30 - 60 cm DBH and radius of 20 m for trees >60 cm DBH). In Plot 5 and Plot 6, the diameter at breast height (DBH) was higher compared to Plot 1, Plot 2, Plot 3 and Plot 4 (Table 2). Plot 5 and Plot 6 are in a wet area, specifically in the Ngambali forest. Forcons, Plot 1, Plot 2, Plot 3 and Plot 4 are in an area that has as table ecosystem (the under growth is airy and litter is thick).
Figure 2 shows that the average of carbon stock for above-ground carbon (AGB) in six plots of Iboubikro and Ngambali was 170.6726667 (t C ha−1). The stocks of carbon about Above-ground biomass (AGB) in Iboubikro forest was 204.693 (t C ha−1) and carbon stock for above ground (AGB) in Ngambali forest was 136.6523333 (tC ha−1). Figure 2 shows that in this study, high quantity of carbon was obtained in Plot 3 (223.192 t C ha−1), followed by Plot 1 (197.147 t C ha−1), then Plot 2 (193.74 t C ha−1). The small quantity of carbon was stored in the Plot 4 (99.574 t C ha−1). The area state of Plot 5 and Plot 6 in Ngambali forest is a swamp with high percent of
![]()
Table 1. Distribution of trees in study area by diameter class.
T: Total of trees; NTC*: Number of trees with diameter class 10 - 30 cm of DBH; NTC**: Number of trees with diameter class 30 - 60 cm of DBH; NTC***: Number of trees with diameter class >60 cm of DBH.
![]()
Table 2. Structuring of study area and distribution of carbon stocks.
*: Carbon stock of Above-ground biomass (t C ha−1), **: Nest area (m2), ***: Diameter at Breast Height (cm).
humidity in the soil. Also, there are many Millettia laurentii in this forest that is characterized by growth and development of spectacular as reported by Makany, (1976) . Trees in full bloom (Millettia laurentii) attract attention because they possess purplish blue flowers. Table 2 shows that, the state area of Plot 1, Plot 2, Plot 3 and Plot 4 is normal because Plot 1, Plot 2, Plot 3 and Plot 4 are in an ecosystem that has not under gone a disturbance of human action. Also, the environment is nothumid and there’s a biological balance of the ecosystems. It there’s a good functioning of ecosystems in these plots in Lesio-louna forest. Table 2 shows that, the state area of Plot 5 and Plot 6 in Ngambali site (gallery forest) is swamp. Plot 5 and Plot 6 are in a swampy area. However, this study shows that, the state of plot does not influence the amount of carbon of said plot. But it is especially the nature of the species of the plot, which influences the quantity or stock of carbon. The roots of the trees of Fabaceae family have swellings called nodules that contain nitrogen-fixing bacteria as reported by Fayolle et al. (2013) . There are 52 species recorded in Iboubikro site during the study and 41 species recorded in Ngambali site of Lesio-louna (Table 3). The total number of trees recorded in the study area was 93 species (Table 3). However, from a functional point of view, given the significant differences in carbon stocks, Lesio-louna forest could play an important role in carbon sequestration and could be a carbon sink on all Teke trays, even as across the Central African forest basin (Congo Basin), since different authors have observed the phenomenon of forest growth. Forest growth on savannas Lesio-louna is a fact, because the carbon stocks in biomass of this forest reserve are more than important in above-ground biomass (AGB) compared to below-ground biomass (BGB). In this gallery forest (Ngambali and Iboubikro), there are observation of the progress of the forest to savanna as reported by Makany, (1976) .
Kearsley et al. (2013) about Conventional tree height-diameter relationships significantly overestimate aboveground carbon stocks in the Central Congo Basin find an average aboveground carbon stock of 162 ± 20 mg C ha−1 for intact old-growth forest, which is significantly lower than stocks recorded in the outer regions of the Congo Basin. The best available tree height-diameter relationships derived for Central Africa do not render accurate canopy height estimates for our study area. Aboveground carbon stocks would be overestimated by 24% if these inaccurate relationships were used. The studied forests have a lower stature compared with forests in the outer regions of the basin, which confirms remotely sensed patterns. Relatively few studies to date have quantified the measurement error or model uncertainty associated with the estimates of forest biomass. In one of the comprehensive studies, Chave et al. (2005) assessed the effects of measurement error (stem diameter), model uncertainty associated with height-diameter relationships, and sampling uncertainty on estimates of tropical carbon stock in Panama. They reported that the uncertainty (standard deviation) in the aboveground biomass for individual trees averaged 47% of the estimate, with 31% arising from uncertainty in the allometric model and 16% from measurement error. At the stand level, however, the effect of measurement error was reduced to less than 1%, and the total uncertainty reduced to 10% due to allometric uncertainty and 10% due to sampling uncertainty. In another study, Djomo et al. (2010) propagated uncertainty in carbon stock estimates in tropical forest in Cameroon using the statistical propagation techniques described in Chave et al. (2005) , and reported that uncertainty in allometric equations contributed 30% of the total uncertainty in carbon stock estimates. These estimates may have overestimated the uncertainty due to allometric models. Another limitation of these studies is that they have focused on tropical forests. Previous studies have tended to focus on uncertainty in carbon stock estimates, rather than uncertainty in carbon change over time. Carbon change is arguably the more important of the two metrics as it is the basis for United Nations Framework Convention on Climate Change (UNFCCC) reporting, including programs such as REDD+ (Pelletier et al. 2012) . We expected that model uncertainty is likely to be less important for carbon change estimates provided that the same allometric equations are used to calculate carbon stocks at both time periods.
4. Conclusion
The study shows that, the carbon stock in a forest ecosystem is not necessarily influenced by the number of trees of this forest ecosystem. Diameter at breast height (DBH) of a tree is not necessarily related to the carbon stock of the tree, as we have seen in some high diameter at breast height (DBH) trees, but with a low carbon stock. We also recorded some trees with low diameter at breast height (DBH), but with a significant stock of carbon. This research has helped us to understand that in these two gallery forests, Iboubikro forest has a higher carbon stock compared to Ngambali forest. In general, Iboubikro and Ngambali forests of Congo have a higher carbon stock and can participate effectively in mitigating of global climate change.
![]()
![]()
Table 3. Distribution of species by family in six plots of study area.
Acknowledgements
The authors grateful acknowledge Chinese and Congolese governments by China Scholarship Council (CSC), Beijing Forestry University, Université Marien Ngouabi, MDDEFE-REDD+/WRI Project and Lesio-louna Pro- ject for supporting this research.
NOTES
*Corresponding author.