Acute toxicity of Cypermethrin, a synthetic pyrethroid to estuarine clam Katelysia opima (Gmelin) and its effect on oxygen consumption ()
1. Introduction
Water is one of the precious liquid of the natural resources available. A plentiful supply of clean water is essential for survival of human being, plants and animals. The disposal of industrial and agricultural waste directly into the aquatic medium burdens the ecosystem [1] . Environmental pollution is one of the undesirable side effects of industrialization and an important aspect of environmental degradation [2] . The pollutants associated with the industrial effluents are organic matter, inorganic dissolved solids, fertilizers, thermal constituents in the form of heat suspended solids, microorganisms and pathogens. Among these pollutants, organic pollutants decrease the level of dissolved oxygen in the water bodies. The disposal of these waste materials or waste water leads to contamination of rivers, lakes, and chronically affects the flora and fauna [3] . The exotic organic chemicals include surfactant in detergents, pesticides, various industrial products and the decomposition products of other organic compounds. Many of these are non biodegradable and are degraded only at a very slow rate. Some of these compounds have been found to be toxic to fish at very low concentration [4] . Pesticides are spreading over agricultural crops, throughout the year with different formulations and with different modes affect aquatic inhabitants. Presence of pesticide in streams and lakes is largely due to the runoff from agricultural fields and outfall from pesticide manufacturing factories [5] . Pesticides are not highly selective but are generally toxic to many non-target organisms. The aquatic environment is also polluted by pesticides and it leads to many changes in organism physiology [6] . Pesticides are indicated to cause respiratory distress or even failure by affecting respiratory centers of the brain or the tissue involved in breathing. The effect of toxicants on the respiration of fishes and invertebrates has received widespread attention and were reviewed by [7] [8] . Aquatic organisms like prawns, fish, bivalve, crab respire through gills. Such respiratory surfaces frequently encounter harmful pollutant present in water in different forms. These pollutants may lead to the alteration in the normal respiratory area which causes reduction in oxygen consumption and physiological imbalance in the organism [9] .
The activity of animal can be measured in terms of oxygen uptake. Aquatic animals have to pass large quantities of water over their respiratory surface and are subjected to relatively greater risk of exposure to the toxic substances [10] . Depletion in oxygen content occurs in the medium when pesticides, chemicals, sewage and other effluents containing organic matter are discharged into water bodies. Pesticides at sublethal concentrations present in the aquatic environment are too low to cause rapid death directly, but may affect the functioning of the organisms, disrupt normal behavior and reduce the fitness of natural populations. In the aquatic environment, one of the most important manifestations of the toxic action of chemical is the over stimulation or depression of respiratory activity.
The review of literature shows that, there is no adequate information about the effect of pesticide on respiratory metabolism of estuarine clam, Katelysia opima. Hence the present study was carried out to study the alteration in the rate of oxygen consumption in estuarine clam, Katelysia opima after exposure to acute doses of Cypermethrin.
2. Materials and Methods
The estuarine clams, Katelysia opima were collected from Bhatye estuary, Ratnagiri. The animals were immediately brought to the laboratory and acclimatized for 4 - 5 days in laboratory conditions. Medium sized healthy and active bivalves were used for experiments. Clams well acclimatized to the laboratory condition were grouped in tens and kept in plastic containers containing 5 liters filtered estuarine water. Static bioassay tests were conducted for 96 hours by using Cypermethrin (25% EC). For every experiment, a control group of clam was also run simultaneously. For the formulation of test concentration, pilot experiment was conducted and range of concentration selected was such that it resulted in zero to total mortality. The formula used to prepare the pesticide solution is as follows:
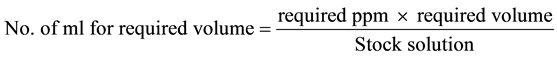
The volume of the container was maintained at 5 L for each. Observations were made at 12, 24, 36, 48, 60, 72, 84 and 96 hours behavioural changes of the clams after introduction into different pesticide concentration were determined.
For the selection of test concentrations, some pilot tests were performed to determine the range of toxicity of the pesticide. The range of concentrations selected was such that it resulted in zero to hundred percent mortality for short term exposures. The LC50 value for each time period was estimated by a regression analysis determined for the log of concentrations and percentage survival of the clam. The percentage mortality in various concentrations at particular period were converted into probit values and plotted against the log of concentrations [11] .
The toxicity tests were repeated three times and LC0 and LC50 values were determined. The regression equation between the log of concentration (X) and probit mortality (Y) were determined statistically for acute toxicity using the formula Y= α + β log (x) and 95% fiducial limits were established according to Finney [12] .
Oxygen consumption experiments were performed in specially designed glass jars of one liter capacity fitted with rubber lid containing inlet and outlet rubber tubes. The marked clams were kept, one in each jar and immediately filled with filtered estuarine water through a siphon and then clamped at both the ends and were kept aside for one hour. Dissolved oxygen was determined by Winkler’s method from the estuarine water [13] . The rate of oxygen consumption of the LC0 and LC50 groups of the clams along with control after every 12 hours time interval was determined. All the values were subjected to statistical analysis. Rate of oxygen consumption was calculated in terms of ml/hr/gm wet weight. Comparing the results with control, the changes in the rate of oxygen consumption from LC0 and LC50. The changes in the rate of oxygen consumption from LC0 and LC50 concentration were statistically calculated [14] . The experiment was repeated three times for confirmation of the results. Such experiments were performed during November to March 2012.
3. Results
During experimental period, clams showed no mortality up to 1.86 ppm. 10% mortality was found at 2.04 ppm.; whereas 20%, 30%, 40%, 50%, 60%, 70% and 80% mortality was found in 2.23, 2.41, 2.60, 2.79, 2.97, 3.16 and 3.53 ppm, respectively at the end of 96 hours (Table 1). The calculated LC50 value was 2.75 ppm and the ob- served LC50 value was 2.79 ppm. The regression equation established was Y = 0.6242 + 9.9484x, the 95% fiducial limit was 1.7632 - 3.7368 ppm for this season.
The measurement of the rate of oxygen consumption in Katelysia opima after acute exposure to Cypermethrin showed significant increase in LC0 group and significant decrease in LC50 group as observed in Table 2.
The control group of clam showed fluctuations in the rate of oxygen consumption between 0.217 ± 0.0022 to 0.326 ± 0.0022 ml/L/hr/gm from zero to 96 hours. In LC0 (1.86 ppm) group, clams exhibited fluctuations in the rate of oxygen consumption between 0.215 ± 0.0027 to 0.376 ± 0.0018 ml/L/hr/gm during zero to 96 hours. There was a considerable increase from 0.215 ± 0.0027 to 0.376 ± 0.0018 ml/L/hr/gm from zero to 60 hours. From 60 hours, there was a considerable decrease up to 0.297 ± 0.0025 ml/L/hr/gm at the end of 96 hours. As compared to control, there was 6.44%, 2.30%, 1.26% and 0.92% (p < 0.001) decrease in oxygen uptake at the end of 84, 96, 72 and zero hours, respectively. There was an increase of 32.39%, 22.29%, 16.77%, 12% and 3.74% at the end of 60, 48, 36, 24 and 12 hours, respectively. In LC50 (2.79 ppm) group, the rate of oxygen consumption fluctuated between 0.174 ± 0.0018 to 0.356 ± 0.0018 ml/L/hr/gm from zero to 96 hours. There was a considerable increase from 0.210 ± 0.0154 to 0.356 ± 0.0018 ml/L/hr/gm at the end of 24 hours. It decreases from 0.247 ± 0.0015 to 0.217 ± 0.0022 from 36 hours to 48 hours and there was a decrease in the rate of oxygen consumption from 0.244 ± 0.0031 to 0.174 ± 0.0018 from 60 to 96 hours. As compared to control, 42.94%, 42.76%, 35.96%, 26.68%,
![]()
Table 1. Regression equations, 95% fiducial limits with LC50 values for Katelysia opima exposed to Cypermethrin.
![]()
Table 2. Rate of oxygen consumption (ml/L/hour/gm. Wet wt.) in Katelysia opima exposed to different concentrations of Cyper- methrin after acute exposure during November to March, 2012.
Values in parenthesis are percent change. ± = S.D. of five trials. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
18.75%, 14.08% and 3.22% (p < 0.001) decrease at the end of 84, 96, 72, 48, 36, 60 and zero hours, respectively. There was 9.53% (p < 0.001) and 1.70% (p < 0.01) increase at the end of 24 and 12 hours respectively.
4. Discussion
In the present study, the control group of clams showed increased oxygen uptake. As compared with control group of clams, LC50 group showed decreased oxygen uptake. The observed decrease is attributed to variation in the volume of water ventilated through the gills, caused by the intermittent closure and opening of the shell valves. Here the main factor responsible for decreased oxygen uptake was coagulation of mucus on gills due to Cypermethrin exposure. Coagulation of mucus causes reduction in the effective transfer of oxygen to internal tissues, adversely affects the absorption of oxygen from the ambient medium. The stiff suppression in the rate of oxygen consumption was probably due to the reduced efficiency of gills. In the present study, considerable mucus secretion was found in lethal concentration during the experimental period. These fluctuations in the oxygen uptake rate in estuarine clams exposed to Cypermethrin are due to variations in gill ventilation rate coupled with the concentration of pollutant in water and the efficiency of assimilation of oxygen via the gills and also the length of time during which the shell valve is closed [15] . In Gafrarium diverticulum suppression of oxygen consumption and rate of filtration was observed after 96 hours Benzene and gear oi-WSF exposure [16] . Reduction inoxygen consumption was reported in LC50 group of fresh water Lamellibranch marginalis from Godavari [17] . Similar observations were observed in various animals at different sub-lethal concentrations of heavy metal and or pesticides [18] - [23] .
To cope with this hypoxia, clams tried to filter more water. High oxygen consumption rate showed by the Clams from LC50 group indicated a high energy demand. As compared to the control group, clams treated with LC0 concentration showed a slight elevation in oxygen consumption after initial 12 hours exposure. These findings relate with the findings of many workers. It was observed that oxygen consumption increased in P. viridis exposed to low concentrations of Ag, while concentrations above 0.01 ppm, oxygen consumption sharply decreased [24] . In L. marginalis pesticide induced increased oxygen uptake was observed. The decrement may be due to the respiratory distress as a consequence of the impairment of oxidative metabolism [25] .
The effect of Cypermethrin showed copious secretion of mucous. The high mucous secretion deposited on the respiratory surface may interfere the rate of oxygen diffusion and therefore oxygen consumption can be de- creased. The bivalves usually try to avoid toxicants and in doing so, they can minimize their metabolic activity. The metabolic stress of the drug may decrease the rate of oxygen consumption. Respiratory inefficiency and ul- timately total respiration breakdown can also be due to the formation of mucous on the respiratory organs in case of pollutant exposure to Bellamya bengalensis [26] . While studying the effect of heavy metal on the histo- logical structure of the gills of crustaceans, the decline in the rate of oxygen consumption was due to the forma- tion of coagulated mucous over the gills and body surface of the crab [27] . The pollutant treated molluscs se- crete mucous in large amount to reduce exposure to environmental stress or pollutant [28] . In the present inves- tigation the decrease in the rate of oxygen consumption in the bivalve treated with Cypermethrin may be due to mucous secretion on body surface, pesticide induced respiratory stress and impaired oxidative metabolism.