1. Introduction
Heavy metal pollution has become a worldwide environmental issue over the last few decades. Chromium is one of the major pollutants present in waste waters from various industrial units such as electroplating, fertilizers, pigments, tanning, mining etc. [1] . Chromium exists in two stable oxidation states, i.e., trivalent Cr (III) and hexavalent Cr(VI) [2] . The latter is highly water soluble and reported to be a potent carcinogen [3] . Prolonged exposure to hexavalent chromium (Cr(VI)) causes serious health hazards such as cancer in the digestive tract and lungs, epigastric pain, nausea, vomiting, severe diarrhoea and hemorrhage [4] . Therefore, Cr(VI) removal is very essential before it is being discharged into the aqueous streams [5] .
Various conventional methods are available for heavy metal removal which is very expensive and not eco- friendly [6] . Biosorption using biomaterials has received considerable attention for detoxification of toxic heavy metals from wastewaters [4] as they possess various merits such as low cost, high efficiency, minimization of chemical and or toxic sludge, regeneration of biosorbent, metal recovery etc. [7] . Fungi are known to tolerate and detoxify metals by several mechanisms and are considered as potential biosorbent for heavy metal removal [8] .
Both living (bioaccumulation) and non-living (biosorption) microbial biomass can act as effective metal sca- vengers [9] . Dead fungal biomass has been used in a number of studies because it is not affected by adverse op- erating conditions and can solve the environment problems of high toxicity [10] . Fungal phenolic polymers and melanins possess many potential metal-binding sites with oxygen-containing groups including carboxyl, hy- droxyl, carbonyl and methoxyl groups being particularly important in biosorption [11] .
Modifications of the cell wall of microorganisms have been explored extensively to increase the sorption effi- ciency of metal ions by microbial biomass using several modifying agents. The treatment varies with the type of biomass and the metal ions to be biosorbed [12] . Different physical methods were used for pretreatment such as boiling, heating, autoclaving and freeze drying or chemical treatments such as, with acids, detergents, alkalis and organic/inorganic chemicals, or a combination of both physical and chemical methods [13] - [17] . These types of pretreatments modify the cell surface which is essential for biosorption either by removing or masking the groups or exposing more metal binding sites [12] .
Alkaline (caustic) treatment could enhance metal binding by biomass. The alkaline treatments, including so- dium hydroxide, potassium hydroxide, alkaline detergents or other alkaline reagents ruptures the cell wall of the microbes and exposes additional functional groups for metal ion binding [18] .
The aim of this study was to investigate the chromium tolerance of 4 Aspergillus spp. and to study the effect of sodium hydroxide pretreatment of Aspergillus spp. on chromium biosorption.
2. Materials and Methods
2.1. Collection of Tannery Effluent
The tannery effluent was collected from the common treatment effluent plant located in Chrompet, Chennai, India.
2.2. Isolation and Identification of Fungi
Potato Dextrose Agar (PDA) was used for culturing fungi from tannery effluent. 100 µl of the effluent was spread on the PDA plate. The inoculated plates were incubated at 27˚C for 5 days. The fungi were identified us- ing Lactophenol cotton blue [19] .
2.3. Screening of Aspergillus Species for Chromium Tolerance
The chromium tolerance of A. terreus, A. tamarii, A. flavus and A. niger was evaluated at various concentrations (1, 3 & 5 mM) of Potassium dichromate (K2Cr2O7) supplemented in PDA and was poured in the petriplates. 6 mm disc of each fungus was inoculated and incubated at 27˚C for 5 days. The culture in PDA without K2Cr2O7 served as control. Reduction of radial growth rate was used as an index for metal tolerance. To compare the heavy metal tolerance of each species, a parallel Index of Tolerance (T.I) was calculated as a percentage value from the ratio:
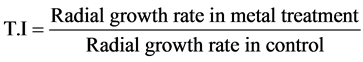
The isolates exhibiting better growth after incubation were considered as chromium tolerant [20] . The toler- ance studies were conducted in duplicates and the mean values were used to compare the tolerance of Aspergil- lus spp.
2.4. Preparation of Biosorbent
2.4.1. Live Biomass
6 mm disc of each fungal culture was transferred to Potato Dextrose (PD) broth and incubated at 27˚C for 5 days. Then it was harvested and washed with distilled water and stored at −20˚C.
2.4.2. Alkali Pretreated Biomass
Live harvested biomass (50 gm) was treated with 0.5 N Sodium hydroxide (NaOH) for 30 min followed by washing with distilled water until the pH of the solution reached 7. The pretreated biomass was autoclaved at 15 lb/inch2 for 20 min. and was dried at 60˚C for 24 hr in hot air oven and powdered using mortar and pestle [21] .
2.5. Biosorption Experiment
0.1 gm of live and alkali pretreated A. terreus, A. tamarii, A. niger, and A. flavus was inoculated into 100 ml metal solution containing 1, 3 & 5 mM of K2Cr207. The flasks were kept on rotary shaker 125 rpm for 18 hr at 30˚C. Then the solution was centrifuged at 10,000 rpm for 15 min. The content of the supernatant was analysed after proper digestion and dilution by atomic absorption spectrophotometer (Perkin elmer 5300 DV). Metal so- lution without biomass addition served as control.
Bioadsorption experiments were carried out in duplicate and average values were used in the analysis. Bioad- sorption capacity, i.e. amount of metal ions (mg) bioadsorbed per gm (dry mass) of biomass was calculated us- ing the following equation:

where Q = mg of metal ion bioadsorbed per gm of biomass, Ci = initial metal ion concentration mg/l, Cf = final metal ion concentration mg/l, m = mass of biomass in the reaction mixture gm, V = volume of the reaction mix- ture (1) [21] .
3. Results and Discussion
In the present study, 23 fungi were isolated from tannery effluent (Table 1). This includes 9 Aspergillus sp., 5 Penicillium sp., Paecilomyces variotii, Trichoderma viride, Graphium sp., Scopulariopsis brevicaulis, Sporoth- rix schenckii, Fusarium oxysporum and Acremonium strictum. Shazia et al. [22] have isolated 19 fungi from heavy metal contaminated soils. Similar to our present study, Aspergillus sp. were frequently (12 out of 19) en- countered than other fungi. Aspergillus sp. were observed to be the most commonly occurring in the heavy metal contaminated soils as also reported by Ahmad et al. [21] and Zafar et al. [23] .
Several Aspergillus species have been used for heavy metal ion adsorption such as A. niger [24] , A. fumigatus [25] , A. niveus [26] , A. versicolor [27] , A. flavus [28] , A. terreus [29] and A. cristatus [30] etc. Sen and Ghosh Dastidar [31] have also studied Aspergillus sp. from industrial wastewater for hexavalent chromium removal. Shazia et al., [22] also reported that Aspergillus isolates were the most resistant to all the metals tested, namely cadmium, copper and nickel.
Hence, in the present investigation, four Aspergillus species viz., Aspergillus terreus, Aspergillus niger, As- pergillus flavus and Aspergillus tamarii were taken under study.
Microbes in metal polluted environments adapt to toxic concentrations of heavy metals and become metal re- sistant [32] . Therefore in the present study, Chromium tolerance of the Aspergillus species was checked using 1, 3 and 5 mM of Potassium dichromate solution where chromium exist as Cr(VI). Growth was observed in 1 mM and 3 mM. No growth was observed in 5 mM. The results were presented in Plate 1. Growth inhibition could have resulted due to chromium toxicity at higher concentration.
Metal tolerance index was calculated for all the 4 Aspergillus sp. (Table 2). For 1 mM Potassium dichromate concentration, the chromium tolerance index of A. terreus and A. niger was same, i.e., 0.5, whereas for A. flavus and A. tamarii, it was 0.3 and 0.2 respectively and for 3 mM Potassium dichromate concentration, chromium to- lerance index for A. terreus, A. niger decreased to 0.3 and for A. flavus, the tolerance index remained same. For A. tamarii also there was a decrease, i.e., it was observed to be 0.01. The variation in degree of tolerance was most probably due to the potential variation in the mechanism of tolerance [33] .
In this study two types of biosorbent of the Aspergillus species were used for Cr(VI) removal. One is the live biomass and the other, is the Alkali pretreated biomass. Then it was used in Cr(VI) biosorption similar to that of Yan and Viraraghavan [18] . Biosorption potential of both alkali pretreated and live biomass of A. terreus, A. tamarii, A. niger and A. flavus was analyzed using metal solution containing K2Cr2O7 (Cr(VI)) at various con- centrations of 1, 3 and 5 mM at pH 7 suggested by Ahmad et al. [21] .
![]()
Table 1. List of fungi isolated from tannery effluent.
![]()
Table 2. Metal tolerance index of Aspergillus species towards hexavalent chromium.
-: No growth.

Plate 1. Screening of chromium tolerance of Aspergillus species: (1) control; (2) 1 mM potassium dichromate; (3) 3 mM potassium dichromate; (4) 5 mM potassium dichromate.
The amount of Cr(VI) removed by live and alkali pretreated A. terreus, A. tamarii, A. flavus and A. niger was given in Figures 1-4 respectively. The results depicted that in all case, Cr(VI) biosorption increased with in- crease in metal ion concentration.
![]()
Figure 1. Comparison of chromium biosorption potential of alkali pretreated and live A. terreus.
![]()
Figure 2. Comparison of chromium biosorption potential of alkali pre- treated and live A. tamari.
![]()
Figure 3. Comparison of chromium biosorption potential of alkali pretreated and live A. flavus.
![]()
Figure 4. Comparison of chromium biosorption potential of alkali pretreated and live A. niger.
At 1 mM concentration of Potassium dichromate (K2Cr2O7), live A. flavus exhibited maximum Cr(VI) removal, i.e., 3.61 mg/g and A. niger showed minimum Cr(VI) removal, i.e., 0.296 mg/g. In alkali pretreated form, maxi- mum Cr(VI) removal was exhibited by A. terreus (32.23 mg/g) whereas A. tamarii showed lowest biosorption (14.83 mg/g). At 3 mM concentration of K2Cr2O7, A. terreus showed maximum Cr(VI) removal, 3.83 mg/g and 38.74 mg/g, whereas A. niger showed minimum Cr(VI) removal, i.e., 1.14 mg/g and 15.71 mg/g in live and al- kali pretreated form respectively. At 5 mM concentration of K2Cr2O7, the amount of Cr(VI) removed by A. ter- reus was higher, i.e., 11.64 mg/g and 42.65 mg/g in live and alkali pretreated form, respectively whereas live A. tamarii exhibited lowest biosorption value, i.e., 2.88 mg/g and in alkali pretreated form, A. niger showed mini- mum Cr(VI) removal, i.e., 17.3 mg/g.
In this study, out of 4 Aspergillus species selected for chromium biosorption, A. terreus exhibited highest biosorption efficiency, in which alkali pretreated form showed maximum biosorption efficiency at higher con- centrations of Potassium dichromate.
Raja Rao and Bhargavi [34] also used sodium hydroxide for pretreating A. niger and studied lead and nickel biosorption using atomic absorption spectrophotometer. The maximum removal of lead was observed around 75% - 80% at pH 6 - 7 with maximum adsorbent dose of 0.2 g/ml. Nickel was observed to have maximum bio- sorption of around 50% - 60% at pH 5 - 8.
Das et al. [35] investigated the effect of sodium hydroxide pretreatment on Cd2+ biosorption capacity of Pleurotus florida. Pretreatment of biomass with NaOH showed maximum increase on biosorption of Cd2+ by approximately three times in comparison with living biomass (from 3.21 to 9.76 mg/g). The reason may be due to the removal of surface impurities, rupture of cell membrane and exposure of available binding sites for metal bioadsorption after pretreatment.
Abdoun-Ouallouche et al. [36] investigated the effect of different pretreatment processes on biosorption ca- pacity of Rhizopus stolonifer biomass to remove lead and mercury. The highest metal uptake values (12.10 mg/g and 9.75 mg/g for Pb and Hg respectively) were obtained by NaOH treated biomass. Chikkhara and Dhankar [37] also used base treated A. niger for Cr(VI) removal and observed 65.28 mg of metal uptake.
In the case of alkali pre-treatment, bioadsorption capacity of Mucor rouxii biomass was significantly enhanced in comparison with autoclaving. Similar enhancement in metal uptake capacity of the fungal biomass regarding alkali pretreatment was recorded by El-Sayed [38] and Das et al. [35] .
In the present study, even though the metal tolerance index of A. terreus and A. niger is more or less similar, A. terreus showed excellent chromium biosorption efficiency compared to the latter. Similar findings were rec- orded by Zafar et al. [23] . Aspergillus sp. 1 which was less tolerant than Aspergillus sp. 2 accumulated Cr and Cu at a higher level suggesting that there was little if any correlation between metal tolerance and biosorption properties of the test fungi.
4. Conclusion
The present study concludes that fungi isolated from tannery effluent have the ability to resist higher concentra- tions of chromium. This may be due to the development of tolerance or adaptation of the fungi to heavy metals. Among the four Aspergillus species, A. terreus exhibited excellent chromium adsorption capacity. Sodium hy- droxide pretreatment improved the biosorption efficiency than that of untreated fungi. After further investiga- tions, sodium hydroxide pretreated A. terreus could be effectively utilized to remove hexavalent chromium from industrial effluents.
Acknowledgements
The authors sincerely thank Dr. Ishari K. Ganesh, Chancellor, Vels University for providing infrastructure facili- ties to carry out this research work. We also thank IIT-SAIF for their help in heavy metal analysis by atomic absorbance spectrophotometer.