Cytotoxic and Anti-Tumour Promoting Activities of Carbazole Alkaloids from Malayan Murraya koenigii (L.) Spreng ()
1. Introduction
Murraya koenigii is a member of Rutaceae family and represented by about 150 genera and 1600 species. 14 species of this genus are known [1] , but only two species can be found in Malaysia; namely Murraya koenigii and Murraya paniculata [2] [3] . Murraya koenigii is a medicinal important herb of Indian origin. It has been widely used as natural flavouring in curries and sources [3] -[5] , and ingredient in traditional medicine formulations [4] -[6] . It has also reported by researchers that carbazole alkaloids possess various biological activities such as antitumor, anti-oxidative, anti-mutagenic, and anti-inflammatory activities [6] -[8] .
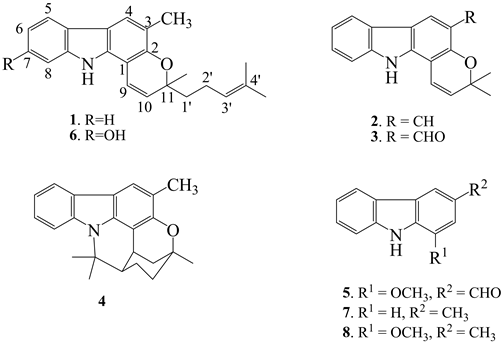
The aim of the present study was to examine the cytotoxic potential and antitumor promoting activity of crude extract and the isolated carbazole alkaloids present in M. koenigii. In our continuing study on leaves, roots and barks of M. koenigii (L.) Spreng, have afforded eight carbazole alkaloids; from bark: mahanimbine 1, girinimbine 2, murrayacine 3, murrayazoline 4 and murrayanine 5; from leaves: mahanimbine 1 and mahanine 6; and from roots: girinimbine 2 murrayanine 5, 3-methylcarbazole 7 and murrayafoline-A 8. Their structures were elucidated by combination of various spectroscopic methods such as 1D and 2D NMR, IR, UV and MS.
2. Experimental
2.1. General Experimental Procedures
Spectra were recorded on the following instruments. The IR spectra were recorded using KBr disc on Perkin Elmer FTIR Spectrophotometer model 1650. UV spectra were recorded on a Shimadzu UV-2100 spectrophotometer. 1H and 13C NMR spectra were obtained on JEOL Spectrometer at 500 MHz with tetramethylsilane (TMS) as the internal standard. Mass spectra were recorded on an AEI-MS 12 instrument. The column chromatography was carried on silica gel (Merck 9385) and Merck silica gel 60 PF254 was used for analytical TLC analysis. Analytical Thin Layer Chromatograph (TLC) was performed on commercially available Merck DC-Plasticfolien TLC plastic sheet precoated witih Keiselgel 60 P254.
2.2. Plant Material
The barks, leaves and roots of Murraya koenigii for this study were collected in
Banting
, Selangor, Malaysia in 1996.
2.3. Extraction and Isolation
The dried bark (
1.1 kg
) of Murraya koenigii were ground into find powder and extracted continuously soaking for three days with petroluem ether, chloroform and methanol. Each extraction was repeated three times. Then the extract was dried on the rotary evaporator. After removal of the solvents, the crude of petroluem ether (
42.2 g
), chloroform (
40.5 g
) and methanol (
40.0 g
) were obtained. After evaporation of the solvent,
15.0 g
of the petroluem ether crude was subjected to column chromatography over silica gel (gradient solvent system; Petroluem ether, CHCl3 and MeOH) yielded five known compounds: mahanimbine (1, 60.1 mg), girinimbine (2, 70.2 mg), murrayacine (3, 4.5 mg), murrayazoline (4, 6.3 mg) and murrayanine (5, 9.9 mg).
The dried leaves (
600 g
) of Murraya koenigii were ground and extracted exhaustively with petroluem ether followed by chloroform and methanol by soxhlet extractor for 17 hours. After evaporation of the solvent,
16.8 g
,
11.2 g
and
15.8 g
of crude extracts, respectively, were obtained.
15.0 g
of petoluem ether crude was subjected to column chromatography over silica gel (gradient solvent system; petroluem ether, chloroform and Methanol) yielded mahanimbine (1,
0.21 g
).
10.0 g
of chloroform crude was subjected over silica gel and yielded mahanine (6, 13.1 mg).
The air-dried and finely grounded roots (
1.1 kg
) of Murraya koenigii were extracted with petroluem ether and methanol for 48 hours for three times. After evaporation of the solvent,
10.0 g
of petoluem ether crude was subjected to column chromatography over silica gel (gradient solvent system; petroluem ether, chlorofom and Methanol) yielded four carbazoles: girinimbine (2,
0.21 g
), murrayanine (5, 30.2 mg), 3-methylcarbazole (7, 8.3 mg) and murrayafoline-A (8,
0.10 g
).
2.4. Cytotoxic Assay
The CEM-SS cell line (T-lymphoblastic leukimia) was obtained from the Natioanl Institutes Cancer, Frederick, Maryland, USA. The cells were cultured and maintained in growth medium as described by Ali et al. [9] . Cytotoxic test were determined by performing the microtitration assay [10] . The tests were performed in 96-well plates, a microtiter plate. Each well was added with
100
mL of varying concentration of crude of Murraya koenigii and the isolated carbazoles prepared from the stock solutions by serial dilution in RPM I - 1640 medium. Subsequently, each well was filled with
100
mL of cell suspension in complete growth medium at 2 × 105 cells/mL.
Controls containing only untreated cells were included for each sample. The assay for each concentration of samples were performed in triplicate and the culture plates were incubated at 37˚C with 5% (v/v) CO2 for three days. The cytotoxicity index used was IC50, which is the concentration that eields 50% inhibition of the cell compared with untreated control. Extracts or isolated compounds which exhibit cytotoxic index (IC50) <
20
mg/mL were considered to possess significant activity.
2.5. Anti-Tumor Promoting Activity Assay
2.5.1
. Treatment of Cells with Samples
Dividing Raji cells/mL in log phase at a density of 5.5 × 105 cells/mL were incubated with
5
mL plant extracts and isolated compounds at various concentrations (1 to
32
mg/mL) in the presence of
10
mL of PMA (
5
mM) and
6
mL of sodium-n-butyrate (
0.5
mM). The cells were incubated for 48 hours in a ninidified incubator at
37 C
with 5% CO
2 in
air. The negative controls consisted of untreated Raji cells treated with the combination of
10
mL of PMA (
5
mM) and
6
mL of sodium-n-butyrate (
0.5
mM).The positive controls consisted of Raji cells treated with the combination of
10
mL of PMA (
5
mM) and
6
mL of sodium-n-butyrate (
0.5
mM).
2.5.2
. Epstein-Barr Virus Activation Assay
The inhibition of Epstein-Barr virus (EBR) activation was used to evaluate in vitro antitumor promoting activity [11] . Raji cells were grown in
10
mL of RPMI 1640 suplemented with 10% fetal calf serum. The cells (5.5 × 105 cells/mL) were maintained in 10 mL of RPMI 1640 medium suplemented witih 10% fetal calf serum containing sodium-n-butyrate (
0.5
mM), phorbol 12-myristate 13-acatate (
5
mM) and test extract (
5
mL) at 37˚C under 5% CO2 for 48 hours using 24 wells plate. After 48 hours, the viability of cells was checked using tryphan blue dye exclusion method. Only those with viability more than 90% were used for this assay. The cells were transferred into appendorf tube and spun down at 1000 rpm for 10 minutes by using a bench centrifuge. The supernatant the cell pellet 1 mL of PBS was added into wash cells and repeated twice. After the second washing, the cells were being spun down at 1000 rpm for 10 minutes. The supernatant was removed and a drop of the cell suspension was added on to a 12 wells test Teflon coated slide (Cooke, USA).
The distribution of the cells was examined under the inverted microscope in order to make sure the drop is confluent and not over dripping. If the spread of the cells was not satisfactory, the dilution of the cells was altered. The slides with the drops of cells were dried in a laminar flow hood. The slides were then fixed in cold acetone for 10 minutes. Cold acetone was obtained by keeping it in −20˚C overnight before fixing the cells. Then, the slides were dried at room temperature and stored in slide boxed at −20˚C for a short period.
Antibody obtained from Nasopharryngeal carcinoma (NPC) patients was used to cover the whole well and incubated at 37˚C for 40 minutes. To avoid drying, the cells were incubated by placing the slides on a wet tissue with cover. After 40 minutes, the slides were washed with Phosphate buffer saline (PBS) twice by dipping in PBS with gentle agitation on a platform shaker for 10 minutes each. Excess PBS was removed using tissue paper by avoiding touching the cells.
Second antibody FITC-conjugated IgG was added by covering the wells. The conjugate used in this study was anti-human IgG flourescein isothiocyanate-conjugated (FITC) (Sigma, USA). Again, the cells were incubated for 40 minutes at
37 C
by putting in a box with water. Then, excess antibody was removed by washing with PBS twice. The slides were dripped in PBS with gentle agitation on platform shaker for 10 minutes each. PBS was removed using tissue paper and avoid touching the cells. Prior checking under UV microscope, 50% glycerol was dropped on the slip (Germany). The slides were then ready for examined under fluorescent microscope.
3. Results and Discussions
Mahanimbine (C23H25NO) 1: white needles, with melting point 93˚C - 95˚C; UV (MeOH) lmax 238, 288 nm; IR (KBr disc) lmax 3338 (N-H), 3312, 2964, 1646 and 1156 (C-O) cm−1; GC-MS m/z 331 (M+). 1H and
13C
NMR see Table 1 and Table 2.
Girinimbine (C18H17NO) 2: colourless crystal, with melting point 171˚C - 173˚C; UV (MeOH) lmax 237 and 287 nm; IR (KBr disc) lmax 3338 (N-H), 1121 (C-O), 742 and
690 cm
−1; GC-MS m/z 263 (M+). 1H and
13C
NMR see Table 1 and Table 2.
Murrayacine (C18H15NO2) 3: greenish needles, with melting point 240˚C - 242˚C; UV (MeOH) lmax 237 and 288 nm; IR (KBr disc) lmax 3222 (N-H), 1188 (C-O) and
1614 cm
−1; GC-MS m/z 277 (M+). 1H and
13C
NMR see Table 1 and Table 2.
Murrayazoline (C23H25NO) 4: yellow crystal, with melting point 265˚C - 267˚C; UV (MeOH) lmax 235 and 287 nm; IR (KBr disc) lmax 2936 (C-H stretching) and 1150 (C-O) cm−1; GC-MS m/z 331 (M+). 1H and
13C
NMR see Table 1 and Table 2.
Murrayanine (C14H11NO2) 5: white needles, with melting point 170˚C - 172˚C; UV (MeOH) lmax 238, 273 and 340 nm; IR (KBr disc) lmax 3167 (N-H) and 1661 (C=O) and 1160 (C-O) cm−1; GC-MS m/z 225 (M+). 1H and
13C
NMR see Table 1 and Table 2.
Mahanine (C23H25NO2) 6: white needles, with melting point 91˚C - 92˚C; UV (MeOH) lmax 235 and 287 nm; IR (KBr disc) lmax 3417 (NH/OH) and 1113 (C-O) cm−1; GC-MS m/z 347 (M+). 1H and
13C
NMR see Table 1 and Table 2.
3-Methylcarbazole (C13H11N) 7: white solid, with melting point 205˚C - 208˚C; UV (MeOH) lmax 235 and 287 nm; IR (KBr disc) lmax 3407 (NH/OH) and
1607 cm
-1; GC-MS m/z 181 (M+). 1H and
13C
NMR see Table 1 and Table 2.
Murrayafoline-A (C23H25NO2) 8: dark brown viscous oil; UV (MeOH) lmax 237 and 287 nm; IR (KBr disc) lmax 3420 (NH/OH) and 1137 (C-O) cm−1; GC-MS m/z 211 (M+). 1H and
13C
NMR see Table 1 and Table 2.
The bark, leaves and roots of M. koenigii were extracted with petroluem ether, chloroform and methanol. The fractionation of the petroluem ether and chloroform extracts followed by column chromatography and TLC yield 8 carbazole alkaloids which were identified as mahanimbine 1, girinimbine 2, murrayacine 3, murrayazoline 4, murrayanine 5, mahanine 6, 3-methylcarbazole 7 and murrayafoline-A 8 by spectroscopic methods (Fig- ure 1). All compounds have showed the similar spectroscopic features with the carbazole alkaloids published in the literature.
Crude extract of the roots and isolated compounds of M. koenigii were screened for cytotoxic activity and antitumor promoting activity. Table 3 showed the inhibition concentration (IC50) of the tested crude extracts and isolated pure compounds that exhibited 50% of T-lymphoblastic leukimia cells (CEM-SS) as compared to the untreated control for 72 hours. All tested samples exhibited significant cytotoxic effects towards CEM-SS cells with IC50 of
3
mg/mL, except girinimbine, in which IC50 of
30
mg/mL.
For the in vitro antitumor promoting activity, all crude extracts and the isolated pure compounds exhibited 100% of inhibition rate. However, most of the samples were cytotoxic towards Raji cells except girinimbine which gave 70.7% viability (Table 4).
![]()
Table 1. 1H NMR [500 MHz, dH (J Hz)] of 1 -
8 in
CDCl3.
![]()
Table 2.
13 C
NMR [125 MHz, dC (J Hz)] of 1 -
8 in
CDCl3.
![]()
Table 3. Cytotoxic activity of crude extracts and isolated pure compounds from the roots of M. koenigii on CEM-SS cell line.
![]()
Table 4. In vitro antitumor promoting activity and Raji cell viability.
Acknowledgements
We gratefully acknowledge the technical support provided by University Pendidikan Sultan Idris and University Putra Malaysia.
NOTES
*Corresponding author.