Total Reflection X-Ray Fluorescence Analysis of Toxic Metals in Fish Tissues ()
1. Introduction
Different kinds of contaminants from agricultural, domestic and industrial sources are increasingly being released into different water bodies and can modify their water quality so it is unsuitable for different purposes such as irrigation, aquaculture, recreation, human consumption, etc. [1] . The pollution of the aquatic ecosystems with heavy metals has become a worldwide problem because they are persistent and can produce a range of toxic effects in aquatic organisms [2] . Fishes are often at the top of the aquatic food chain and may concentrate large amounts of some metals from the water [3] . Furthermore, fish is one of the most indicative factors in freshwater systems, for the estimation of trace metal pollution and risk potential of human consumption [4] . Heavy metals are taken up through different organs of the fish because of the affinity between them. In this process, many of these heavy metals are concentrating at different levels in different organs at the fish body [5] . Hence, it is important to determine the concentrations of heavy metals in commercial fishes in order to evaluate the possible risk of fish consumption.
The grass carp (Ctenopharyngodon idella) is an herbivorous, freshwater fish species of family Cyprinidae, and the only species of the genus Ctenopharyngodon. It feeds on a wide range of aquatic vegetation, and is capable of consuming 40% - 300% of their body mass per day of plant material depending on their age and size. Fry consume planktonic crustaceans, rotifers, and insect larvae, while adults are completely vegetarian. Grass carpare used worldwide for control of aquatic vegetation as well as an important food fish [6] .
The aim of this study was to evaluate the concentration of Cr, Cu, Zn, Hg and Pb in gills, liver, kidney and muscle of grass carp (Ctenopharygodon idella) from Tepuxtepec Dam, Michoacán. This body water is supplied mainly by the Lerma River, which is receiving municipal wastewater, agricultural and industrial discharges, this water contains a large amount of organic and inorganic contaminants that enter the body of water in contact with the biota [7] . Finally, the results obtained were compared with the permissible limits (MPL) established by current Mexican law and the FAO/WHO [8] [9] .
2. Material and Methods
2.1. Study Area
The Tepuxtepec dam, giving rise to the Middle Basin of the Lerma River, is situated in the State of Michoacán, Mexico (20˚02' north latitude and 100˚13' west longitude) (Figure 1). It has a storage capacity of 585 h∙m3 in 57 km2 is the eighth dam water uptake capacity in the State of Michoacán. This reservoir was built for the purpose of generating energy through hydroelectric Lerma and control of Lerma River floods and as a secondary activity is done growing grass carp (Ctenopharygodon idella), under an extensive production system, which depend approximately 230 families who are organized in 6 regions of production and marketing [10] .
2.2. Sampling Collection
In this study 60 grass carp were collected at six production regions in the Tepuxtepec Dam (Figure 1). Ten specimens were randomly sampled in all regions. Grass carp samples were labeled, store on ice and the same day transported to the laboratory for further treatment and analysis.
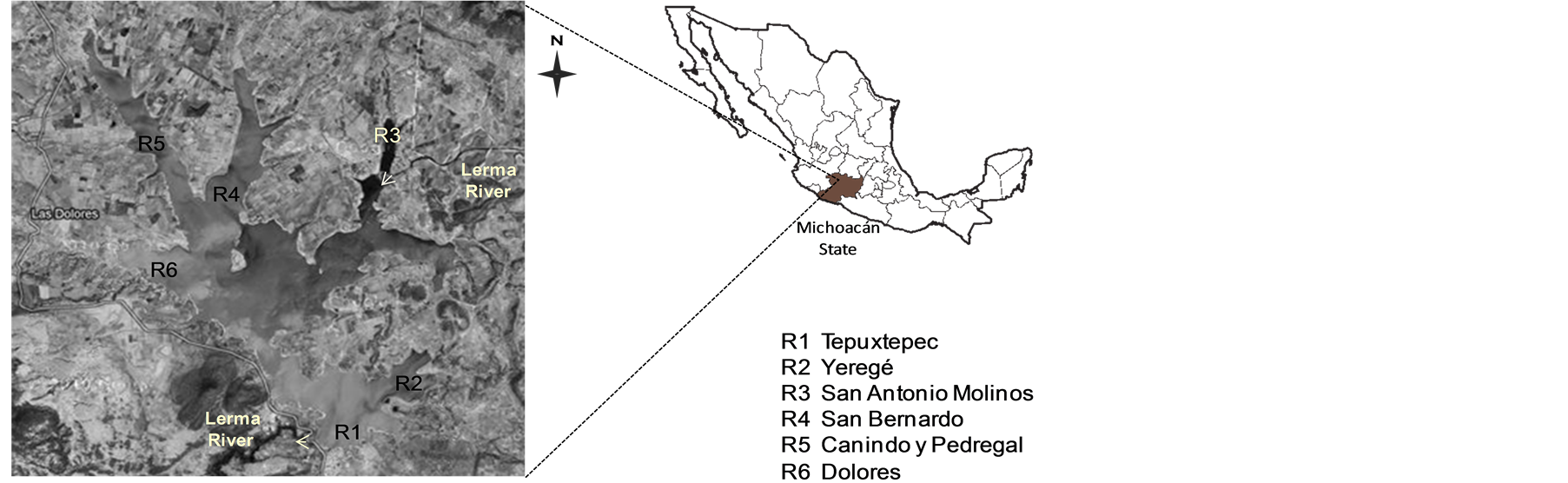
Figure 1. Localization of the Tepuxtepec Dam, Michoacán, Mexico and sampling regions of grass carp (Ctenopharygodon idella).
2.3. Sample Preparation
The grass carp samples were dissected to separate tissues (gills, liver, kidney and muscle) according to FAO methods [11] . Tissues samples were washed thoroughly in running tap water to avoid any surface contamination and then rinsed with de-ionized water. The cleaned grass carp tissues were freeze-dried, ground, sifted to 200 mesh and homogenized. 0.5 g subsample of each region was putted in a closed digestion vessel and digested in a microwave oven (Mars-X, CEM Corporation) according to the 5BI-8 method for “Fish tissue”. The digested sample was transferred into a volumetric flask, the reaction vessels were washed out with de-ionized water and the flask volume made up to a final volume of 25 mL. 100 μL of Yttrium standard solution (Merck) of 10 mg/mL was added as internal standard to 1 mL subsample of the digested samples and mixed. After that, 20 µL of the digested tissue sample was deposited on a silicon quartz glass holder [12] . Only supra pure grade quality chemicals were used.
2.4. Sample Analysis
X Ray Fluorescence is a nuclear analytical technique which has the advantage to be multi-elemental, fast and economical. The TX 2000 X-Ray Spectrometer manufactured by Ital Structures was used in order to obtain the elemental concentration. A primary fine focus beam provided by the X-Ray tube with a molybdenum anode is monochromatised and is directed to the sample at a glancing angle less than the critical angle. The tube was operated at 40 kV and 30 mA. The fluorescent X-Rays derived from the sample were detected with a solid state lithium-drifted silicon detector of 20 mm2 front area, cooled with liquid nitrogen. The energy resolution (FWHM) of the Si (Li) detector was 140 eV for Mn Kα and its beryllium window was 8 µm thick.
Three replicates were prepared for each sample in order to evaluate the reproducibility of measurements. All samples were excited for 500 s. Heavy metal concentration and detection limits were determined by means of the software “EDXRF32-Ital Structures” using the theoretical “Sensitivities with internal standard” method. It is based on the sensitivity values of the different elements. For the analysis of the spectra and metal quantification, the software EDXRF32 was used [13] . Detection limit (DL) listed in Table 1 were calculated with the Equation (1) [14] :
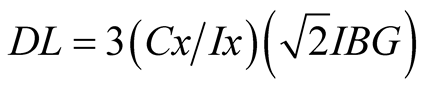 (1)
(1)
where DL: Detection limit (minimum detectable mass mg/Kg); Cx: Analyte concentration (mg/Kg); Ix: Analyte net intensity (counts) and IBG: Background intensity (counts).
2.5. Statistical Analysis
Data were processed by the Statgraphics Plus 5 program in order to obtain the average values, standard deviations and confidence limits. The Statgraphics software allowed investigation of the existence of a normal distribution for the data values. The analysis of variance (ANOVA) was applied to data values when normal distribution was observed, and the Kruskal-Wallis test was used when no normal distribution law was observed, in order to deduce spatial distributions for heavy metals in the Tepuxtepec Dam. The statistical methods were performed with a 95% confidence interval (CI; α = 0.05).

Table 1. Comparison between measured and certified elements concentration for the reference material IAEA MA-A-1 (TM) Copepod homogenate.
SD: Standard deviation; RE: Recovery; DL: Detection limit.
3. Results and Discussion
In order to evaluate the accuracy and reproducibility of the analytical results, three sub-samples of the reference material “IAEA MA-A-1 (TM): Copepod homogenate” from the International Atomic Energy Agency (IAEA) were analyzed. The elemental concentrations in the samples are shown in Table1 As it can be seen, the measured concentrations were consistent with the certified values. Accuracy being measured as the percentage of recoveries (% RE) after the acid digestion (ratio between values measured and certified in the reference material), was higher than 93% and the percentage relative error was from lower than 2% to 13%. Detection limits (DL) for the experimental conditions are also shown.
Maximum, minimum and mean concentration, of the analysed metals in the grass carp tissues are given in the Table2 Concentrations of Cr, Ni, Cu, Zn, Hg and Pb, considered like toxic metals in aquatic environment, varied between <0.03 and 817 mg/Kg, being highest for the zinc and lowest for the mercury and lead.
Analyzing the results it is observed that zinc presented the highest concentration followed Cu, Cr, Ni, Pb and Hg. The results confirm the differences of heavy metal accumulation in the different tissues. In this study heavy metal accumulation order was, Kidney > Gills > Liver > Muscle. On the other hand, target organs, such as liver, gonads, kidney and gills, are metabolically active tissues and accumulate heavy metals of higher levels, as was observed many experimental and field studies [15] [16] , in this study it was found that kidney and gills were the main accumulators of Ni, Zn and Pb; Cr is accumulates in liver and kidney; Cu is accumulates mainly in liver while Hg goes to gills and muscle (Figure 2).

Table 2. Content of Cr, Ni, Cu, Zn, Hg and Pb in grass carp (Ctenopharygodon idella) tissues from Tepuxtepec Dam. Concentration in mg/Kg.
MPL: Maximum permissible limit, NR: Not reported, NA: Not applicable; FAO/WHO, (2004) Comission of Codex Alimentarius. ALINORM 04/27/18; SS, Health Department (1995). NOM: Official Mexican Norm. NOM-027-SSA1-1995.
The high accumulation of Zn could certainly be based on specific metabolism process and coenzyme catalyzed reactions involving zinc taking placed in kidney [17] . Zinc also acts as a catalyst in metal biomolecules bound to aminoacids side chains containing N, O and/or sulfur donor legends to form tetrahedral zinc metalloproteins and metalloenzymes in kidney tissue [18] [19] .
The liver is often considered a good monitor of water pollution with metals since their concentrations are proportional to those present in the aquatic environment. Liver is a target organ of accumulation for many metals, because of its strong irrigation and excretion function. The liver tissue is highly active in the uptake and storage of heavy metals. It is well known that large amount of metallothionein induction occurs in the liver tissue of fishes [20] .
The gills constitute a multifunctional organ (respiration, ion and acid-base regulation, nitrogenous waste excretion, etc.) accounting for over 50% of the total surface area of the animal. Gills are uptake site of waterborne ions, where metal concentrations increase especially at the beginning of exposure, before the metal enters other parts of organism, the reason for high metal concentrations in the gills could be due to the metal complexing with the mucus that is impossible to remove completely from the lamellae before analysis [20] .
Many studies reported that on a number of fish species, which show that muscle is not an active tissue in accumulating heavy metals [15] [21] -[23] . Heavy metal analysis in edible parts (muscle) indicated that, only Hg and Cr were higher than the limits for human consumption in edible parts; however, the monitoring of other metals is important because they are considered toxic to humans and also by the influence of wastewater discharges could exceed the limits for human consumption in edible parts [8] [9] .
Furthermore the accumulation of heavy metals in fishes may induce changes in biochemical metabolism and other induced stress: lead and mercury belong to the group of non-essential and toxic metals, implying no known function in biochemical processes; copper and zinc are both micro-nutrient and toxicant, however Zn is about five-fold less toxic than Cu, and Zn uptake at the gills appears to be a normal branchial function occurring via the chloride cells The gills are an important site for the entry of heavy metals that provokes lesions and gill damage while higher accumulation in liver may alter the levels of various biochemical parameters causing severe liver damage [15] .
Figure 3 shows the metal concentrations by sampling region obtained in this work, in general the element concentrations in the fish tissues were found to decrease in the sequence: R3 > R1 > R2 > R4 > R6 > R5. Statistical differences (α ≤ 0.05) in the metal concentrations, between samples taken in the sampling regions 1, 2 and
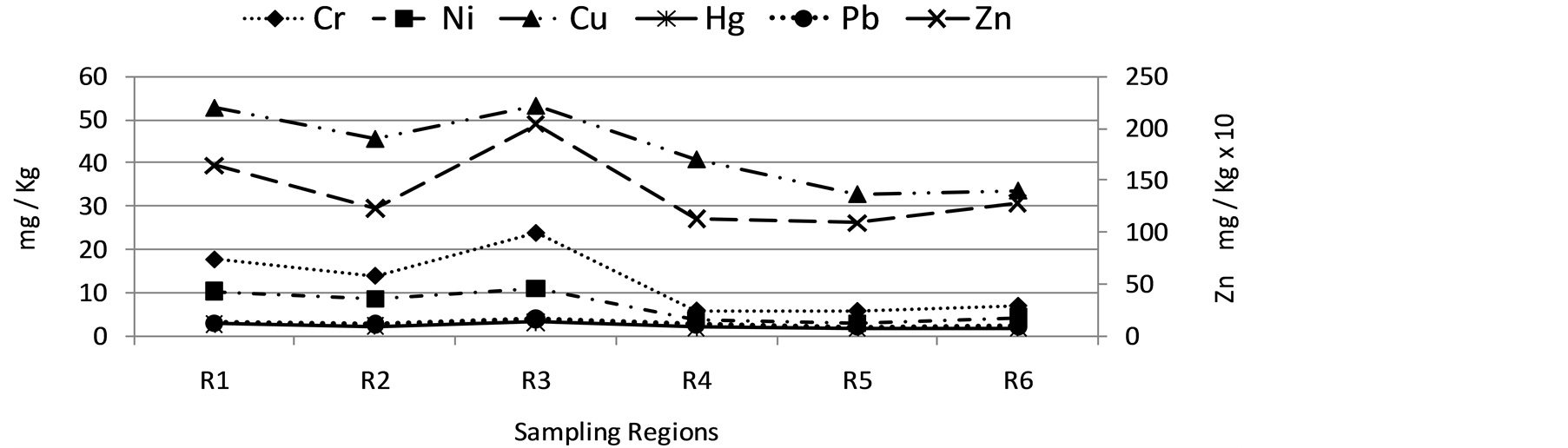
Figure 3. Sum of metal concentration in fish production regions from tepuxtepec dam. Concentration in mg/kg.
3 (spatial variation) were observed respect to the others.
It is general accepted that heavy metal uptake occurs mainly from water, food and sediment however, the efficiency of metal uptake from contaminated water and food may differ in relation to ecological needs, metabolism, and the contamination gradients of water, food and sediment as well as other factors such as age, weight, pH, temperature, etc. [24] .
The presence of metals in this body of water is influenced by the contribution of the Lerma River, which is the main tributary, it presents a high degree of contamination by metals and on the other hand, metals such as Cu, Zn and Hg can be from agricultural runoff, therefore the high levels of metal in fish tissues from sampling regions 1, 2 and 3 may be due to these fish production regions are closer to the inner course Lerma River.
4. Conclusions
The accumulation of toxic metals varied among grass carp (Ctenopharyngodon idella) tissues. Results showed that metal accumulation in the gills, liver and kidney was higher than in muscle, except for mercury.
The concentration of Hg and Cr in muscle, edible tissue, exceeded slightly the maximum permissible limit established by the FAO/WHO and the Mexican Legislation and could pose a health risk to consumers of grass carp from Tepuxtepec Dam.
Higher accumulation of metals in the gills, liver and kidney may be considered a primary signal of metal exposure and can be affect the aquatic life of the fresh water fish.
Heavy metals accumulate mainly in metabolic organs, such as liver and kidney and can be provokes damage in the grass carp (Ctenopharyngodon idella) metabolism.
Most of the data concentrations in the fish tissues of the sampling regions 3, 1 and 2 were higher than concentrations in the other four sampling regions due to the contribution of the Lerma River.
TXRF was used to measure a toxic metal in fish tissues, which had the ability to accumulate them and therefore could be used as a tool for future monitoring programs, to evaluate the metal pollution of the Tepuxtepec Dam.
The accuracy related to the sample preparation and the measurement with the TXRF equipment was very good, which was demonstrated by the use of the standard reference material. In relation to the reproducibility, the results show a very good standard deviation.
The Total Reflection X-ray Fluorescence Spectrometry applied to biomonitoring and toxicology studies offers reliable information about elements considered like toxic by the aquatic life.
NOTES
*Corresponding author.