1. Introduction
Tribe Anthémidées includes 109 genus and 1740 species [1] . The phylogenetic studies have confused the names of species Anthemideae, including genus Chamaemelum, Ormenis and Cladanthus [2] .
Genus Cladanthus is clearly related to the genera Anthemis and Chamaemelum and most species were previously included in the last genus. However, a molecular study of Chamaemelum Mill. and related genus showed that traditionally marked as Chamaemelum is paraphyletic [2] .
Cladanthus is a small genus of five species, all native to the Mediterranean region and south-west Europe [3] .
Cladanthus mixtus (L.) Oberpr. and Vogt, wild Moroccan chamomile, is an aromatic plant known as the vernacular name “Hellâla” [4] , and the newly adopted scientific, including the synonyms Anthemis mixta L., Chamomilla mixta Grenier et Godron, Chamaemelum mixtum (L.) All., Chamaemelum mixtum (L.) All. var. mixtum, Chamaemelum mixtum var. aureum (Durieu) Benedi, Chamaemelum mixtum var. glabrescens (Maire) Benedi, Ormenis aurea Durieu, Ormenis mixta (L.) Dumort. subsp. mixta, Ormenis mixta subsp. aurea (Durieu) Batt., Ormenis mixta subsp. multicaulis (Braun-Blanq. & Maire) Maire, Ormenis mixta var. glabrescens Maire [5] .
The Moroccan chamomile, or simple-leaved chamomile, is a tall spontaneous annual plant, fragrant, 1 m in height, with the peripheral ligulate flowers which are white, a golden yellow at the base and the central florets are tubular; achenes are all identical, devoid of pappus three coasts visible on the internal face, smooth outside [6] [7] .
Moroccan Chamomile widespread in Algeria, Morocco, northern and eastern part of the Mediterranean basin [7] , grows in sandy soils in northern Morocco along the Atlantic coast [8] . It is usually found in woodlands, fields and pastures sandy and stony plain and low mountains [9] .
The infusion of Moroccan chamomile leaves and flowers is used in Moroccan traditional medicine for treatment of different ailments [4] [10] .
Moroccan Chamomile is mainly used for the extraction of essential oil of camphor odor; Morocco is the leading provider in the international market; it is sought in cosmetics, medicine and especially in perfumery [11] -[14] .
In Morocco, wild Chamomile recommended as an anxiolytic and rebalancing the central nervous system, is recommended in nervous breakdowns, for shortcomings liver and stomach light and colibacillaires colitis [12] .
The essential oil of Cladanthus mixtus is active in vitro against Escherichia coli, Bacillus subtilis, Staphylococcus aureus and Micrococcus luteus and fungi Penicillium parasiticus, Aspergillus niger and Trametes pini [15] .
Extract of Cladanthus mixtus inhibits the corrosion of steel in acidic medium. A critical concentration of extract provides the maximum protection [16] .
The plants used in the phyto-pharmaceutical preparations are obtained mainly from the natural growing areas. With the increase in the demand for the extract of these plants, the plants are being overexploited, threatening the survival of many species. Also, many medicinal plant species are disappearing at an alarming rate due to rapid agricultural and urban development, uncontrolled deforestation, and indiscriminate collection. Advanced biotechnological methods of culturing plant cells and tissues should provide new means for conserving and rapidly propagating valuable, rare, and endangered medicinal plants.
Although Cladanthus mixtus presents a significant medicinal and aromatic value, no study focuses on the micropropagation of this species; in vitro culture is the subject of a new study. So we are interested in the vegetative propagation by in vitro culture (micropropagation) of chamomile Morocco: C. mixtus L.
The aim of this paper is to test the effect of different culture media on in vitro seed germination of Cladanthus mixtus (L.), and micropropagation, multiplication and rooting of seedling germination.
2. Materials and Methods
2.1. Plant Material and Sterilization Method
Achenes of Cladanthus mixtus (L.) were provided by INRA Kenitra (Morrocco) under the name Ormenis mixta (L.) Dumort. They were collected from the Maamoura (Morrocco) in 2007.
Achenes are sorted and cleared the rest of the capitulum. They are put in transparent plastic bags and stocked in the dark at room temperature.
Sterilization was performed according to the following protocol: The seeds were submerged in 7% (v/v) calcium hypochlorite for 15 min with additional of three drops of Tween 20, then immersed in 0.1% (v/v) mercuric chloride (HgCl2) for 1 min. and finally rinsed three times (5, 10 and 15 min) with sterile distilled water to remove traces of chlorine.
2.2. Effect of Basal Media on in Vitro Seed Germination
The sterilized seeds was inoculated into sterilized culture into Petri dishes (9 mm) containing 20 ml of different basal media. Thirty seeds were incubated for each treatment and repeated three times, and the seedlings used in subsequent experiments.
The basal media screened, to identify the suitable medium for seed germination were: Ghautheret (1959) [17] (Table 1) and gelled distilled water.
All media were supplemented with 3% sucrose and gelled with 0.7% agar Bacteriological and adjusted to a pH of 5.6 - 5.8 with NaOH and HCl prior to autoclaving at 120˚C for 20 min. All the cultures were incubated at 25˚C under white fluorescent light intensity (800 lux) with a 16 hours photoperiod and 80% relative humidity.
The percentage (%) of seeds germination was visually monitored on each hour, and it was recorded in Figure 1. The seeds considered to be germinated by the emergence of radicles from the seeds [18] . Percentage of seed germination was calculated according to the following equation (adapted from [19] ):

2.3. Effet of Basic Culture Media on Plantlet Development from Shoot Tips from Cladanthus mixtus (L.)
The young seedlings used of 2 - 3 mm were obtained from in vitro germination of seeds (2 leaf stage cotyledonary) of 100 seedlings. They are disposed vertically to the surface of a culture medium containing the macroelements, microelements and vitamins of Murashige and Skoog (1962), myo-inositol (meso-inositol) 100 mg/l, 3% sucrose (Table 2) and solidified with 0.7% bacteriological agar type E.

Figure 1. Effect of medium composition on the germination of seeds Cladanthus mixthus L.

Table 1. Macroelements of Gautheret (1959) used during germination of seeds of Cladanthus mixtus L.

Table 2. Vitamins and sugars MS medium (1962).
Transplanting is done every 30 days, three clones A (small size), B (medium size) and C (long size) were selected from the 9th transplant. The development of the culture medium was carried out on the clone C (long size).
To determine the most favorable to the growth of explants nutrient conditions we chose settings the salt solutions of MS: Murashige and Skoog (1962) [20] , SD: Sahah and Dalal (1978) [21] , SH: Schenk and Hildebrant (1972) [22] , MSm: Murashing and Skoog modified (Badoc, 1982) [23] , and B5: Gamborg and Eveleigh (1968) [24] , (Table 3), with microelements and vitamins of Murashige and Skoog (1962), myo-inositol (meso-inositol) 100 mg/l, agar (0.7%) and 3% sucrose, were tested on the apex of cladanthus mixtus L. (clone A).
These macronutrients are different by their nitrogen (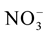 and
and ) and potassium content (Table 4). After 30 days in culture the response was evaluated. The results presented in Table 5, and with examples in Figure 2 and Figure 3, Numbers of shoots, root as well as shoot height and root length, percentage of hypérhydrie and callogenesis were determined after 4 week of culture.
) and potassium content (Table 4). After 30 days in culture the response was evaluated. The results presented in Table 5, and with examples in Figure 2 and Figure 3, Numbers of shoots, root as well as shoot height and root length, percentage of hypérhydrie and callogenesis were determined after 4 week of culture.
2.4. Statistical Analysis
Thirty explants were used per treatment and the experiment was repeated twice. Data collected in all experiments were analyzed by SPSS version 17 and subjected to analysis of variance (ANOVA). The mean differences were tested using Duncan multiple range test (DMRT) with significant value of P < 0.05.
3. Results and Discussion
3.1. Asymbiotic Seed Germination
The disinfecting method was 100% successful in eliminating any seed contamination.
Germination tests are performed under well-defined conditions where all factors are controlled. The germination of Cladanthus mixtus was carried on distilled gelled water and Ghautheret (1959) medium. Trace output is shown in the following Figure 1:
The two basal medium which has been tried with different nutritional composition, gelled distilled water After 33 hours, 100% of the seeds germinated, as considered by emission of the first radical in gelled water.
The maximum rate of germination (100%) is reached just after 34 hours in the medium containing the Gautheret (1959) medium.
The similarity of results can be explained by the ability of achenes Cladanthus mixtus to germinate in different environments.
Seeds of C. mixtus exposed to gelled distilled water medium showed higher germination percentage than for exposure to Gautheret (1959) medium.
This varied response in seed germination in different media might be due to the media compositions as both the media contains very low amount of macro and the micro elements and vitamins compared to Gautheret (1959) and gelled distilled water.
There is no significant effect of the composition of the medium on the germination of seeds Cladanthus mixthus L. So seeds germination does not require specific nutrient solutions to germinate, and Gautheret (1959) positively influenced seed germination and subsequent seedlings development (Figure 1).

Table 3. Macroelements composition (mg/l and mM) of different media used during cultivation of shoot of Cladanthus mixtus (L.)

Table 4. Ionic composition (mEq/l) of five macro-solutions tested.

Table 5. Effects of five standard macrosalt formulations on shoot growth, rooting, and percentage of hypérhydrie and callogenesis of the wild chamomile Morocco: Cladanthus mixtus L. after 30 days. Numbers of shoots, root as well as shoot height and root length, percentage of hyperhydrie and callogenesis.
Means followed by the same letter along rows are not significantly different by Duncan’s test (P ≤ 0.05). MS: Murashige and Skoog (1962); SD: Shah and Dalal (1980); B5: Gamborg and Eveleigh (1968); MSm: Murashige and Skoog modified (Badoc, 1982); SH: Schenk and Hildebran (1972).
 (a)
(a)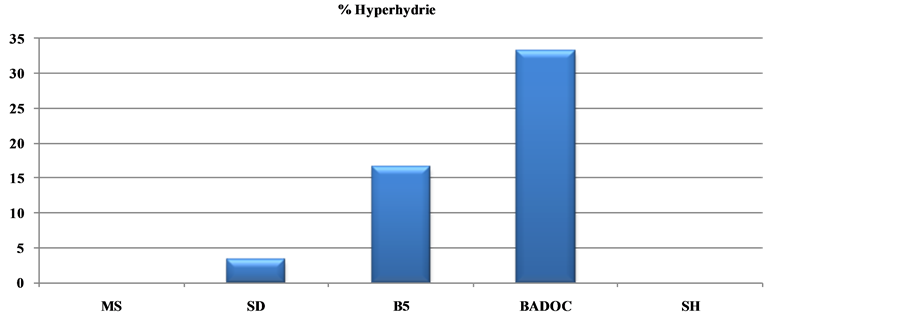 (b)
(b) (c)
(c)
Figure 2. Influence of five macroelements on development, growth and rooting (a), the hyperhydrie (b) and the callus formation (c) of shoot (n = 30) collected explants of long clone (A) cladanthus mixtus L., after 4 weeks of culture.
3.2. Effet of Culture Media on Plantlet Development from Shoot Tips
We tested the effect of the mineral solution on explants of Morocco wild chamomile; five standard macrosalt formulations MS (1962), SD (1978), SH (1972), Badoc (1982) and B5 (1968) were tested for their effect on shoot multiplication; the results are shown in Table 5, Figure 2 and Figure 3.
Statistically significant differences (P ≤ 0.05) were observed in shoot multiplication from explants cultured in five multiplication media containing different macroelements (Table 5).
Significant differences on shoot number between media were obtained in five multiplication media, with the highest multiplication rate obtained on MS medium. However, shoot number did not significantly increase in other media, as observed in MS medium (Figure 2(a)).
On the MS medium, the mean number of shoots per explants achieved 2.60 with a length of 2.75 cm, the mean number of roots achieved 3.33 per explants with a length of 2.42 cm, and the percentage of hyperhydrie and callus is absent (Figure 2(b), Figure 3(a)).
However, a significant reduction on the mean numbers of shoots, roots as well as shoot height and root length, was observed in other media as compared to MS medium.
Among the five solutions macroelements tested (Table 5), those of B5 and MSm prove harmful to the explants; they induce a higher percentage of callus (100%) (Figure 2(c)) and the percentage of hyperhydrie is 16.33% and 33.33% respectively (Figure 3(c)).
MS, B5 and MSm media do not induce callus against in the midium SD representing 30% of callus which will reach 100% in the SH medium (Figure 3(b)).
MS and SH media do not induce hyperhydric plants against by the SD medium which causes a low percentage of hyperhydrie (3.33%), which increases to 16.33% on B5 and 33.33% on MSm medium.
So according to our results macronutrients MS (1962) is more favorable to the multiplication, growth and rooting of shoots of Cladanthus mixtus L. and is used for the following tested growth regulators.
The success of in vitro culture of plant species is influenced by several factors including the culture medium [25] [26] ; we can say that it is the concentration of nitrate that gave the difference between the five culture media. The decrease of N and K in other media and especially MSm (Badoc) results in a low shoot elongation of clone Studied.
The difference between the number and length of the shoots and roots of the clone A in five culture media can be explained by the difference between the concentrations of these minerals elements.
This difference is explained by [27] which showed that the MS (1962) medium is mainly characterized by very high nitrogen content that the third party is brought in reduced form ( ); this chemical element is the fundamental component of the living matter.
); this chemical element is the fundamental component of the living matter.
The presence of a high concentration of potassium (K+) in the MS medium plays a role in the induction of organogenesis, especially for the neoformation of buds, stem elongation and the production of biological materials.
These same results were observed with [28] -[36] , who have successfully observed a micropropagation of Matricaria recutita and d’Anthémis nobilis L. on Murashige and Skoog (1962) medium, with a very high percentage of shoots.
Although more than 50 different devised media formulations have been used for the in vitro culture of tissues of various plant species [37] [38] , the formulation described by Murashige and Skoog (1962) (MS medium) is most commonly used, often with relatively minor changes ([39] -[46] ).
This explains the ability of this species to grow better in the MS (1962) medium as another medium.
NOTES
*Corresponding author.