1. Introduction
As more and more attention is paid to environmental protection and the related regulations are getting stricter, people are in great demand of clean gasoline, diesel oil and advanced lubricating oil. Thus, as a technology for producing petroleum products of high quality, n-heptane isomerization is attracting people’s concern. N-heptane isomerization plays an important role especially in enhancing front-end octane of gasoline and improving viscosity-temperature resistance of diesel [1] . SAPO-11 molecular sieve has been widely used in oil refining and chemical engineering industry, such as catalytic cracking, hydrocracking, alkylating of aromatic hydrocarbon with branched chains, iso-dewaxing and light olefin polymerization, etc. [2] . As active component, precious metal (such as Pt and Pd) is always carried by SAPO-11 molecular sieve. Nevertheless, due to the expensiveness of precious metal and its rigorous demand of sulfur content of raw material, the application is limited. In recent years, people start to use base metal (Ni, Mo and W) as an alternative of precious metal, and apply it in alkane isomerization. However, this catalyst is still limited in experimental observations because of its low alkane conversion rate and isomeric selectivity.
In this study, SAPO-11 was prepared by hydrothermal crystallization as carrier, and then active component Ni was dipped onto the molecular sieve to get Ni/TiO2-SAPO-11 catalyst. The catalytic performance was evaluated by catalyzing n-heptane isomerization in continuous flow fixed bed reactor.
2. Experimental
Principal chemical reagent: According to hydrothermal crystallization, pseudo-boehmite (Fu Rao industry and trade Co. Ltd, Zi Bo), phosphoric acid (Zhen Hua chemical Co. Ltd, Qing Dao) and acidic silica sol (Sai Wei technology and trade Co. Ltd) were utilized as resources of aluminum, phosphorus and silicon respectively. The temple reagent of SAPO-11 was made by diisopropylamine (Jian Bei organic chemical Co. Ltd, Shang Hai) and di-n-propylamine (Jian Bei organic chemical Co. Ltd, Shang Hai). SAPO-11 supported Ni was provided by nickel nitrate (Hua Dong reagent work, Shen Yang). The other chemical reagents used in this study are tetrabutyl titanate (Sinopharm Chemical Reagent Co. Ltd), n-butyl alcohol (Da Mao chemical reagent work, Tian Jin), n-heptane (Hua Dong reagent work, Shen Yang). All reagents are analytical pure.
Catalyst preparation: First, SAPO-11 molecular sieve was prepared by hydrothermal crystallization [3] . The original molecular sieve powder was calcinated at 550˚C for 4 h to remove the temple reagent. TiO2-SAPO-11 was prepared by sol-gel method. After being incipiently impregnated with an aqueous solution of Ni(NO3)2, Ni/TiO2-SAPO-11 catalyst was obtained [4] .
Catalyst characterization crystal: The XRD patterns of crystal phase analysis were obtained on Science D/max-2200 x-ray diffractometer (Japan Co., Ltd) using Cu-Kα radiation, tube voltage 40 V, tube current 40 mA, scan rate 40˚/min, stepping rate 0.02˚/s and scan range 20˚ ~ 80˚. The specific surface area and pore diameter distribution of catalyst were measured on Konta NVOA/2000e (USA) specific surface and pore diameter analyzer, with N2 as adsorbate.
Catalyst property evaluation: The isomerization of n-heptane was carried out on a continuous flow fixed-bed reactor and the products were identified by FID of gas chromatograph. Catalytic reactivity was evaluated through analyzing the conversion of reactants and isomerization product selectivity. The conversion (X) of n-heptane and the selectivity (S) to isoheptane are calculated as follows:
 . (1)
. (1)
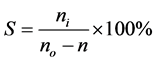 . (2)
. (2)
where no and n denote the moles of n-heptane in the feed and the product respectively; ni denotes the mole of n-heptane converted to isoheptane.
3. Results and Discussion
3.1. Effect of Ni Content of Catalyst on Catalytic Performance
Figure 1 shows the effect of Ni/TiO2-SAPO-11 with different Ni content on n-heptane isomerization conversion rate and selectivity. Metal function improves in the wake of the increasing of Ni capacity. Before Ni capacity reaches 2%, n-heptane isomerization selectivity increases from 86.21% to 88.97%. However, the selectivity decreases gradually when Ni capacity is over 3%. This is ascribed to metal function has exceeded the demand of B acid, which leads to an abundant of cleavage reaction.
The accruing amount of metal activity—which increases with the capacity—improves catalyst’s ability of hydrogenation/dehydrogenation, thus more transitional molecules which avail cleavage or isomerization are supplied for further reaction, as a result n-heptane conversion is enhanced. With the addition of Ni capacity catalyst activity increases and conversion rises up constantly. According to the analyzes of experimental data above, we can conclude that the most proper Ni capacity is 2%, with conversion and selectivity that are

Figure 1. Effect of varied Ni content on conversion and selectivity of Ni/ TiO2-SAPO-11 catalyzed n-heptane isomerization. ■—conversion; ●—selectivity.
achieved 40.97% and 88.97% respectively. All the follow-up experiments were carried out based on NiTiO2-SAPO-11 catalyst with Ni capacity of 2%.
3.2. Effect of Calcined Temperature of TiO2-SAPO-11 on Catalyze Performance
Effect of TiO2-SAPO-11 calcined temperature on conversion and selectivity of Ni/TiO2-SAPO-11 catalyzed n-heptane isomerization is given in Figure 2. As been showed in the figure, when TiO2-SAPO-11 calcined temperature is 400˚C ~ 700˚C, conversion of n-heptane firstly rises up and then falls down. When calcined temperature is up to 500˚C, conversion of n-heptane increases while the selectivity of isoheptane is the highest. The highest conversion of n-heptane appears at 600˚C, with the lowest isoheptane selectivity. This is because under 600˚C, Ni/TiO2-SAPO-11 produced from TiO2-SAPO-11 has the biggest acidity, which leads to more cleavage reactions and less isomerization selectivity. As the calcined temperature getting higher, the specific surface area and activity of Ni/TiO2-SAPO-11 keep on decreasing. Hence, the most appropriate TiO2-SAPO-11 calcined temperature is 500˚C.
3.3. The Effect of TiO2 Percentage on Catalytic Performance
The effect of Ni/TiO2-SAPO-11 with different TiO2 content on conversion and selectivity of n-heptane isomerization are showed in Figure 3.
As we can see from Figure 3, both the conversion and the selectivity of catalyst decrease as TiO2 percentage increases. The reason is that TiO2 and SAPO-11 can form composite support together, then the specific surface area of SAPO-11 becomes smaller, as a result the chance for n-heptane and catalyst to contact with each other reduces. According to the experimental data above, when catalyst contains 20% of TiO2, we can get the best catalytic performance, with isomerization selectivity of 88.97%, conversion of 40.94%.
3.4. Catalysts Characterization
XRD characterization: Figure 4 is the XRD pattern of Ni/TiO2-SAPO-11 catalysts with different Ni contents. In the figure, the characteristic peaks of SAPO molecular sieve appear at 16.08˚, 21.86˚, 22.46˚, 29.80˚ and are sharp and with high strength, which means the sample’s crystal texture owns a high degree of crystallinity. There are no visible remarkable peaks due to metallic Ni, so we can conclude that the crystal form of catalyst
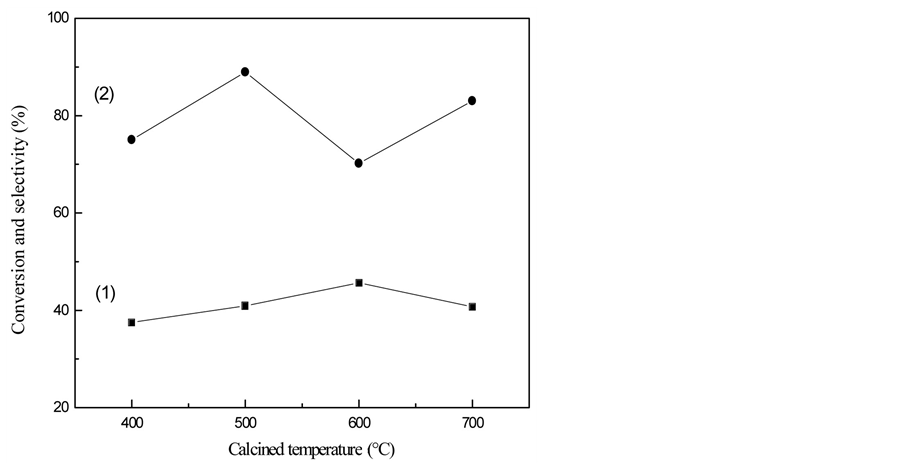
Figure 2. Effect of calcined temperature on conversion and selectivity of Ni/TiO2-SAPO-11 catalyzed n-heptane isomerization. ■—conversion; ●—selectivity.

Figure 3. Effect of TiO2 content on Ni/TiO2-SAPO-11 catalyst performance with 2% Ni content. ■—conversion; ●—selectivity.
remained stable. However, with the increasing Ni capacity, the strength of SAPO-11 peaks recede and get wider. This is possibly due to the enrichment of metal on molecular surface, the preparation process of catalyst and the calcinations.
BET characterization: The data of Ni/TiO2-SAPO-11 specific surface areas under different calcined temperatures are listed in Table1
According to the data of Table 1, as calcined temperature of TiO2-SAPO-11 increases, due to particles cluster, the structure of Ni/TiO2-SAPO-11 collapses and the specific surface area reduces.

Figure 4. XRD figure of Ni/TiO2-SAPO-11 catalyst with different Ni content. 1—Ni content 10%; 2—Ni content 20%; 3—Ni content 30%; 4—Ni content 40%; 5—Ni content 20%; ●—SAPO-11; ▼—TiO2.

Table 1 .Specific surface area of Ni/TiO2-SAPO-11 under different calcined temperature.
4. Conclusion
The best conditions for preparation of Ni/TiO2-SAPO-11 catalyst are determined by experiments. The results show that the optimal preparing conditions are 20% of TiO2 mass fraction, 2% of Ni mass fraction and calcining at 500˚C. The n-heptane conversion and isoheptane selectivity of Ni/TiO2-SAPO-11 could reach up to 40.94% and 88.97%, respectively. The catalyst is simple for preparing, environmental friendly and with highly potential in industrial application.
Acknowledgements
The authors gratefully acknowledge the support from the Provincial Key Laboratory of Oil & Gas Chemical Technology of Daqing. This study was supported by scientific research fund of Heilongjiang provincial education department (12521063).