1. Introduction
Marine atmosphere with its high level of salinity and humidity is very corrosive, and the effects of corrosion are allegedly responsible for 30% of failures on ships and other marine equipment. It has been estimated that the total cost of marine corrosion worldwide is between $50 - 80 billion every year [1] . The coating industry is almost 40% of the total direct costs, and the largest portion of this cost (88%) is attributed to organic coatings [2] .
The corrosion rate in an open natural system is controlled by the diffusion rate of oxygen from the bulk solution to the steel surface and the composition of the carbon steel that is being attacked has no effect on rates [3] . The level of dissolved salts of the exposure environment had an effect on the properties of coatings, initially the corrosion rates are higher and are at least 2.5 times the subsequent steady state rate that begins earlier than one month following exposure according to some studies [4] .
Accidents involving ships that carry environmentally dangerous cargo, such as oil tankers, can have severe consequences for the environment. In many accidents, the ship structure had been weakened by corrosion [5] - [7] . Maritime safety and the prevention of accidents require a sound ship structure. As such, the prevention of corrosion is a key priority for safe shipping. Today’s coating systems work well and have a sufficient lifetime when they are applied according to the coating producer’s specifications [8] - [10] .
There are organic and metallic protective coatings, other common methods for preventing and controlling corrosion include: corrosion-resistant alloys, plastics and polymers; corrosion inhibitors; and cathodic protection. Coatings used for maintenance purposes on vessels will account for 65%, and 20% for offshore drilling and platforms [11] . The effects of corrosion are expensive costs to vessel, structural and equipment operations, com- pounded by the financial penalties on breakdowns, outages, and repairs. These problems have challenged the industry for years, but it needs to remain proactive in preventing in controlling marine corrosion. The many types of marine corrosion, their possible interaction, and the need to review the entire system when considering changes highlight the importance of tackling the problem through design, selection of materials, construction, use and maintenance. Sharing knowledge of research findings and the expertise of those developing modern engineering systems are vital to controlling the damaging effects of the marine environment [12] [13] .
Paints used in marine environments are usually constituted by a polymeric resin (typically epoxy, alkyd or polyurethane), organic solvents and wide variety of organic and inorganic additives (as pigments, biocide, fillers, etc.) [14] - [17] . Active anticorrosive pigments like metallic chromates passivate metal surface by covering it with an oxide film. However, these agents are preferably kept out of the environment due to the toxicity of heavy metals.
On the other hand, metals like zinc, which are usually employed as anticorrosive additive in epoxy paints, prevent discharge of current from substratum (naval steel) to electrolyte by electrochemical attachment of a less passive anode, i.e. it covers the paint into a sacrificial coating. In recent years a new generation of paints that employ water as solvent has emerged. However, their anticorrosive performance is poor with respect to that of the organic-based paints they intend to replace. This is a critical problem for the shipbuilding industry, which requires high specifications due to the aggressive marine environment. In spite of this, promising results have been reported for different ecological water-based paints that incorporate zinc phosphates as non-contaminant anticorrosive additive. Thus, the entry of water through the coating produces a zinc film that passivates the metallic substratum. However, once the anticorrosive additive has been exhausted, there is a complete loss of the protective properties of the paint because of its high porosity making difficult the practical use of this type of paints in aggressive environments. Regarding to the barrier protection, this consists in protecting the metal surface blocking the passage of oxygen and water. Usually, the protection by barrier effect provided by a coating is evaluated using immersion assays, in which both the volume of water and oxygen that enters into the system and the rate of penetration are measured [18] [19] . The structure of the polymers is essential to decide the extent of permeation through it. Indeed, the relationship between structure of polymers and their permeability has been successfully exploited in applications like membranes and packaging. However, in coating applications the presence of pigments and additives can alter the porosity and adherence of the films modifying their protective action. For instance, pigments reduce the porosity of the coatings; the smaller the pigment particles, the lower the porosity.
2. Experimental Procedure
2.1. Materials
Three different coatings were painted (Table 1) on 5 mm thickness steel plates. The chemical composition of the steel substrate is shown in Table 2. Prior to coating the substrates were prepared according to ISO 8501- 4:2006 [11] [20] .
![]()
Table 2. Chemical composition of the steel substrate (wt %).
2.2. Experimental Tests
2.2.1. Adhesion Test
Adhesion Pull-Off and Tape (X-Cut test + Cross Cut test) tests were measured according to ASTM D 4541 and ASTM D 3359 standard tests respectively (ISO 4624 and ISO 2409 tests). The results of the adhesion test are illustrated in Table 3.
2.2.2. Salt Spray Test
To evaluate surface layer for the three different painted samples, unpainted sample as reference Gravimetric test and Visual and optical inspection were done. Atmospheric marine test (ASTM B287-62) is used (Figure 1), where chloride atmosphere attack the samples. Chloride atmosphere mainly comes from the reaction between acetic acid and Sodium chloride with different concentration (as standard), at 40˚C and aeration. Duration of the test is about 54 hours; each 2 hrs a weight change (ΔW) was estimated. Finally, the change in the weight was plotted versus time. Visual inspection and optical microscopy was done to investigate the surface layer attack and the formed corrosion product. As well as, pitting initiation and propagation was inspected.
2.2.3. DC Electrochemical Polarization Tests
All tests were carried out using Autolab Potentiostat/Galvanostat (PGSTAT 30) specified for electrochemical measurements Figure 2. A conventional three-electrode cell, (a single compartment-glass cell of 250 ml capacity was used with stainless steel as counter electrode). The working electrodes were the coated specimens with an exposed area of 0.785 cm2. All potentials were measured with respect to saturated calomel electrode (SCE). The electrolyte consisted of 3.5% NaCl solution made up from distilled water and reagent grade NaCl (Fisher scientific). Experiments were carried out at room temperature. Linear polarization technique was carried out by subjecting the working electrode to a potential range of 20 mV below and above corrosion potential (Ecorr) at a scan rate of 0.0125 mV/sec. The current within this range varied linearly with applied potential. The polarization resistance, Rp (Ω), was determined from the slope of the plots of the applied potential against the measured current. The corrosion current Icorr (A) can be calculated using the Stern-Geary relationship:
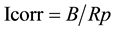
where

where, βa and βc are the anodic and cathodic Tafel constants in (v/decade).
By this technique, controlled potential scan (0.5 mv/s) was typically applied to the sample starting at Ecorr and extending in both anodic and cathodic directions for a few hundred millivolts (about ± 250 mv from Ecorr). When the resultant potential-current data were plotted as shown in Figure 3, it characteristically exhibited linear regions on both anodic and cathodic branches. The slope of these linear regions in v/decade of current is known as the Tafel constants βa and βc respectively. The corrosion rate C.R. in mpy (millimeter per year) can then be calculated from Faradays law by:

![]()
Figure 2. Autolab potentiostat/galvanostat.
![]()
Figure 3. Results of potential-current data.
where, EW and d are the equivalent weight (g) and density (g/cm3) of steel and icorr is the corrosion current density (mA/cm2) that obtained from icorr = Icorr/exposed area.
3. Results and Discussion
The results of the adhesion tests are shown in Table 3.
DC electrochemical polarization test results are shown in Table 4 and the corrosion rate of the samples are shown in Figure 4.
In general coating improves the corrosion resistance of uncoated steel. It is clear that coat number 2 and 3 show much lower corrosion resistance then coat number 1.
The weight change (mg/cm2) of the three samples coated with different types of paint was measured in chloride atmosphere at 40˚C for about 54 hrs. These results are plotted in Figure 5. As seen one can drive a conclusion that sample 2 could withstand 54 hours without aggressive attack, the other two samples 1 and 3 show significant changes in the weight gain. Higher weight gain was obtained for samples 3 and 1 than sample 2. In comparison to the reference sample (without surface coating), lower weight gain was recorded. The values of the corrosion rates in mm/yr after 54 hours from the experiment time is calculated from the relation (Corrosion rate = K W/ρAt) as shown in Table 5. Where W is the weight loss after exposure time t; ρ and A represent the density and exposed specimen area, respectively, and K is a constant, its magnitude depending on the system of units used (K = 87.6 and W, ρ, A, and t are specified in units of milligrams, grams per cubic centimeter, square inches, and hours, respectively).
To support our result, visual inspection as well as optical micrographs was carried out during exposure time. In general, all the samples illustrate pitting behaviour with different degree according to its surface layer, weakness and strength. However, micrographs of sample 2 represent few pitting attack in comparison to sample 1 and 3 and also the reference. Although the pitting attack of the surface layer (paint), one can drive a final conclusion that sample surface without coating is aggressively attacked by pits. Larger and wider pits are formed on that surface. In all cases dark brown corrosion product was formed around the pits. Some selected micrographs are shown in Figure 6 for sample 1, 2 and 3 and also for comparison, reference sample (without coating) after 54 hrs.
![]()
Figure 4. Corrosion rate of coated and uncoated samples.
![]()
Table 4. DC electrochemical polarization test results.
![]()
Figure 5. Salt spray corrosion rates corrosion.
4. Conclusions
From the present experimental study and the obtained results, it can be concluded that:
1) Polymeric coating for steel structure for marine application is essential to reduce salt water corrosion. A corrosion rate reduction of about 77% was realized for coated surfaces compared to uncoated steel surface.
2) Minimum corrosion rate of 0.8 mm/year was exhibited by the coat “HEMPALIN ENAML 52140” over a salt spray test duration of 54 hours.
3) Optical micrographs have shown that oxidation and pitting dominate the corroded surfaces.
NOTES
*Corresponding author.