1. Introduction
Several pharmaceutical compounds have been detected in domestic wastewater, natural aquatic systems and groundwater in many countries around the world [1] [2] . Studies show that these substances have been detected inaqueous systems in concentrations of ng∙L−1 to µg∙L−1 [3] [4] . Sources of these contaminants are mainly the domestic wastewater due to the excretion of non-metabolized drugs by human or animal urine and feces [3] [5] . Ibuprofen (IBF) is one of the most consumed medicaments worldwide, mainly due to its use to the treatment of pain and fever relieve. The non-metabolized IBF is excreted by urine or feces and reaches the domestic wastewater. Studies show that more than 70% of the IBF can be removed by biological treatment [6] . Despite the high biological removal, the remaining IBF can cause environmental problems [7] . Therefore, alternative and more effective treatments need to be studied to remove IBF and other pharmaceuticals from wastewaters.
The application of Advanced Oxidation Processes (AOPs) has shown promising results to remove pharmaceutical pollutants in aqueous systems [8] [9] . The AOPs are chemical processes that have in common the generation of highly reactive hydroxyl radicals (·OH), which is responsible to initiate the oxidation process of several compounds that can be found in water, soil and air [10] -[13] . The heterogeneous photocatalytic process using TiO2/UV is one of the most investigated AOPs. UV with wavelength below 380 nm has enough energy to generate eletron/hole (e−/h+) pairs at the titanium dioxide surface. The e−/h+ can react with adsorbed water or oxygen and generate the ·OH. The AOPs have several advantages when compared to traditional wastewater treatments, and one of them is the possibility to totally mineralize the organic compound to H2O and inorganic acids. However, total mineralization is not always achieved and some AOPs by-products are reposted to be more toxic than the original compound [1] [14] [15] . Therefore, it is also important to study the toxicity of the AOP treated effluent in order to safely dispose it to the environment.
Studies of IBF degradation using TiO2/UV are reported in the literature; however most of them use high concentrations of the pharmaceutical compound and when low IBF concentrations are reported, there is an absence of toxicological assessment [8] [9] [16] [17] .
The aim of this work is to study the degradation of IBF in the presence of TiO2/UV in low concentration and investigate the acute toxicity of by-products against Daphnia similis.
2. Methods and Materials
2.1. Chemicals
Ibuprofen, CH3H18O2, purity ≥ 99%, was purchased from Sigma Aldrich. The titanium dioxide, TiO2, used was from Degussa, P-25. Acetonitrile was HPLC grade from Sigma Aldrich. All solutions were prepared using ultrapure water obtained by Milli-Q system.
2.2. Photodegradation Study
The photocatalytic system used consisted in a 125 W Hg vapor lamp positioned at 5 cm distance from the samples solution. The radiation reaching the samples was 10.75 mW∙cm−2 measured by a radiometer Solar Light PMA 2100 Datalogging Radiometer (Solar Ligth Co). A volume of 500 mL of 1.0 mg∙L−1 of IBF solution at pH value of 7.8, containing the appropriated amount of the catalyst, was irradiated for 60 min and the suspension was maintained under aeration using a peristaltic pump (ISMATEC). Samples of 2.0 mL were withdrawn at time intervals of 0, 15, 30, 45 and 60 min. The catalyst was removed by filtration using 0.45 µm membrane filter and the remaining IBF was analyzed by HPLC. All studies were conducted in triplicate and the results shown here is the average of these results.
2.3. HPLC Analysis
High Performance Liquid Cromatography (HPLC) analyses were carried out on Agilent 1200 Infinity Series equipment, using a diode array detector (DAD), C18 eclipse plus column (1.8 µm, 4.6 × 150 mm). The mobile phase consisted of 40% ultra-pure water and 60% acetonitrile, at isocratic mode for 10 min. The flow rate was 1.3 mL∙min−1 at 22˚C. The volume injected was 10 µL and IBF was detected at 222 nm of wavelength.
2.4. Total Organic Carbon Analysis
Total organic carbon (TOC) analyses were performed using the TOC equipment from Analytik Jena, Multi N/C 2100S.
2.5. Toxicity Test against Daphnia similis
The toxicity test with Daphnia similis were carried out following the protocol procedure of the Brazilian Association of Technical Standards (NBR 12713/04) [18] . The newborn test organisms with 6 to 24 h of age were exposed to 30 mL of samples, withdrawn from the degradation study (0, 15, 30, 45 and 60 min). The cultures were maintained in an incubator at 22˚C with controlled photoperiod of 12 h in the light and 12 h in the dark. After 24 h and 48 h, dead organisms were counted and the mortality percentage was calculated.
3. Results and Discussion
3.1. Ibuprofen Determination by HPLC
The literature shows that for the determination of some anti-inflammatory compounds by chromatographic method there is a need to use buffer solutions [13] [19] [20] . The present developed method used to identify and quantify IBF does not use buffer solution and the present method was validated as shown.
The calibration curves for IBF showed a linear regression correlation coefficient of 0.9999 and 0.9908, to concentration of IBF from 0.125 mg∙L−1 to 1.0 mg∙L−1 and from 1.0 mg∙L−1 to 10.0 mg∙L−1, respectively. The detection limit (DL) and quantification limit (QL) to the calibration curve from 0.125 mg∙L−1 to 1.0 mg∙L−1, were calculated using the Equations (1) and (2), respectively [21] [22] :
 (1)
(1)
 (2)
(2)
S is the angular coefficient and s is the estimated standard deviation of the calibration curve. The Microcal Origin® software was used to calculate these values. The DL was 0.0169 mg∙L−1 and QL was 0.0514 mg∙L−1.
3.2. pH Variation
The pH value is an important parameter to consider when heterogeneous reactions are involved.
Results of a pH variation study, from 3 to 9, were shown at Figure 1. The results show that the optimum pH value to degrade IBF was observed at pH 7.8. The pH of zero point of charge (ZPC) for titanium dioxide is reported to be pH 6.25 - 6.4 [23] -[25] . The IBF is a weak acid with pKa value of 4.4. Therefore, at lower pH range, TiO2 will have a positively charged surface as well as the carboxyl group of IBF and charge repulsion will be observed. At higher pH value, TiO2 and IBF will be both negatively charged and electrostatic charge repulsion will be observed. Better conditions are expected at pH close to neutral [8] . All subsequent degradation studies were performed using pH 7.8.
3.3. Photodegradation Study
Studies using TiO2 as photocatalyst are reported since the 1980s [26] [27] . Each study uses a different concentration of catalyst. Therefore, a study of the optimization of the TiO2 amount was performed varying the concentration from 4 to 20 mg∙L−1. The main objective of this study was to obtain the minimum catalyst concentration necessary to efficiently degrade IBF in a short irradiation time. Figure 2 shows the results and it can be observed that at the first 15 min. most of the IBF were degraded no matter what TiO2 concentration was used. After 30 min of irradiation the degradation efficiency decreased probably due to the saturation of the catalyst surface [8] . The optimum IBF degradation efficiency was observed using 20 mg∙L−1 of TiO2, reaching more than 98% degradation at 30 min of irradiation. All subsequent degradation studies were conducted using 20 mg∙L−1 of the catalyst.
Control experiments were also studied. Results showed that although adsorption of IBF onto TiO2 surface was observed, its contribution to the removal of this compound was less than 5% after 60 min. of contact.These results are in agreement with Mendez-Arriaga et al. [9] . Studies with only aeration of the IBF solution did not remove this compound from the aqueous system. Direct photolysis was also investigated and less than 5% of IBF was removed from solution after 60 min of UV irradiation.
Most of the research work studying the removal of IBF uses a high concentration of this compound, reaching up to 200 mg∙L−1 [7] -[9] . In the present work lower IBF concentrations were used trying to be closer to those detected in the contaminated aquatic systems, but at the same time, above the detection limit and quantification limit of the procedure discussed in 3.1. Results of this study are presented in Figure 3. It can be observed that decreasing the IBF concentration, the degradation rate is faster, reaching more than 95% removal in 15 min,

Figure 1. Comparison of the pH variation in the efficiency of the photodegradation study of 1 mg∙L−1 of IBF using 4 mg∙L−1 of TiO2. Irradiation source125 W Hg vapor lamp, aerated solution; total solution volume: 500 mL.

Figure 2. Comparison of the photodegradation efficiency of 1 mg∙L−1 of IBF with variation of the catalyst concentration. Irradiation source125 W Hg vapor lamp, aerated solution; pH 7.8; total solution volume: 500 mL.
when IBF concentration is less than 0.5 mg∙L−1, while at the same time period, only 55% IBF was removed when the initial concentration was 5.0 mg∙L−1. Nevertheless, total removal was always reached after 60 min of irradiation with all of the IBF concentrations investigated. These results validate the hypothesis that if the degradation is observed at higher concentration, similar response can be expected to the treatment of the effluents that contain lower concentration of the compound than the studied one.
The removal of the initial compound does not indicate that the total mineralization was achieved; therefore, a study of the total organic carbon (TOC) quantification was also necessary. Using 5 mg∙L−1 of IBF and 20 mg∙L−1 of TiO2, the TOC value decreased in 50% after 30 min. of irradiation and maintained at this value until 60 min of reaction. These results show that complete mineralization is not achieved even though IBF removal was total. Similar results were observed by other researchers [7] -[9] . The chromatograms show the appearance of other signals, probably due to the generation of intermediates (results not shown in this work).
Literature shows that it is common to generate intermediates during AOP and that many of those intermediates can be more toxic than the initial compound [7] [28] . Therefore, a toxicity study of the irradiated solutions was conducted.
3.4. Toxicity Assessment
Most of the reports study the toxicity of the initial compound and little attention is given to the possible generated by-products [29] -[32] . As mentioned before, IBF is not totally mineralized and therefore some by-products have been generated during TiO2/UV irradiation study. The possible toxicity of the IBF and its generated byproducts were investigated against Daphnia similis. Figure 4 shows the results of this test. It can be observed that as the irradiation time increases, the toxicity of the sample solution also increases. Sample containing only IBF, at time zero of irradiation, showed to be non-toxic to this organism, probably due to its lower concentration used to the test, 1 mg∙L−1. Reference [33] showed that IBF can be toxic to Daphnia similis but at higher concentrations, around 50 mg∙L−1, with an acute effect to 50% of the test organisms.
After 15 min irradiation, the samples showed toxic effects to the organisms reaching up to 80% mortality when sample with 60 min irradiation was studied. These results show that probably the by-products generated during AOP treatment are the responsible to the increase in the toxicity of the solution. This behavior was also reported in the literature [9] using Vibrio fischery inhibition test. The toxicity can be related to the hydroxilated
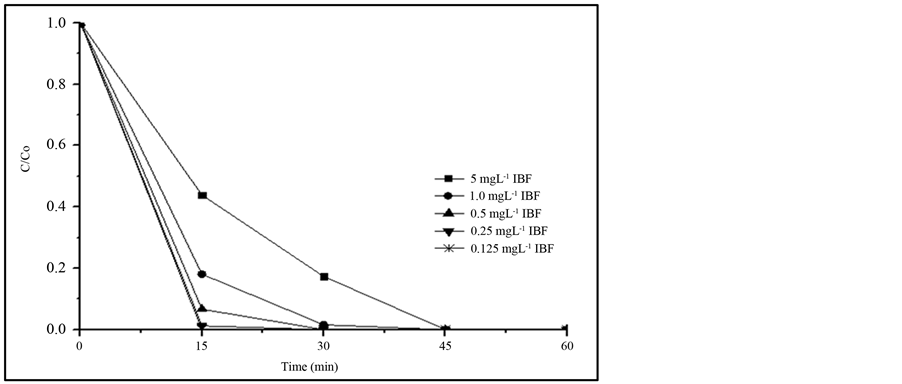
Figure 3. Comparison of the photodegradation efficiency at different IBF initial concentrations, using 20 mg∙L−1 TiO2 (P25). Irradiation source: 125 W Hg vapor lamp, aerated solution; pH 7.8; total solution volume: 500 mL.
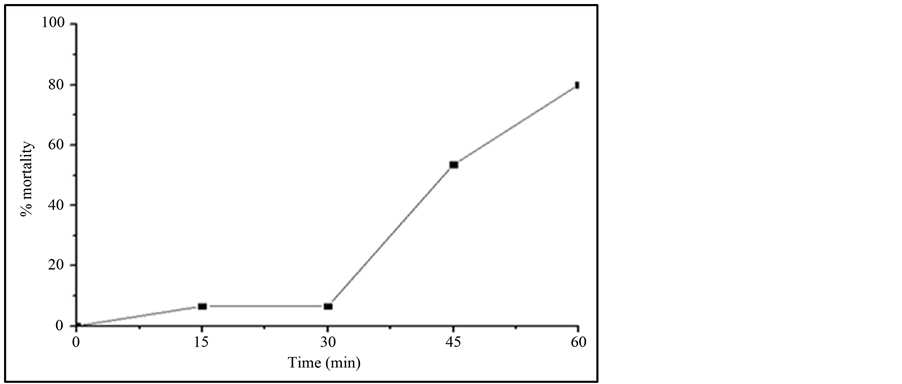
Figure 4. Mortality of Daphnia similis after 48 h of exposure to the samples withdrawn from the TiO2/UV irradiation study, using 1 mg∙L−1 IBF and 20 mg∙L−1 TiO2. pH of IBF solution: 7.8, Irradiation source: 125 W Hg vapor lamp, aerated solution; total solution volume: 500 mL.
by-products generated, which was reported by [9] . Therefore, it is important to verify that the total mineralization is reached and not only the total removal of the initial compound. When these by-products cannot be analyzed, TOC values can be used as indirect tool to verify the mineralization.
4. Conclusion
The photocatalytic degradation of IBF in aqueous solution in presence of TiO2 and UV radiation was studied. Under the studied parameters, neither the direct photolysis nor the adsorption of IBF onto TiO2 surface was observed. Total IBF removal can be achieved at less than 60 min of irradiation, however total mineralization was not observed. The by-products generated during TiO2/UV treatment showed to be more toxic to Daphnia similis than the original compound. These results show the importance to study not only the removal of the target compound by also monitor the by-products generated. Some of them, even at very low concentration, can be more toxic than the original organic compound. These observations show that the AOP treated effluents require attention if using in combination with biological treatment processes, as well as if discharged direct to the environment.
Acknowledgements
The authors would like to thank FAPEMIG and Rede Mineira de Química (RQ-MG) for the financial support.
NOTES
*Corresponding author.