Role of Cathepsin G in the Degradation of Glyceraldehyde-3-Phosphate Dehydrogenase Triggered by 4-Hydroxy-2-Nonenal in U937 Cells ()
1. Introduction
Under oxidative stress, membrane lipids are oxidized to various reactive aldehydes [1] . These aldehydes are considered to be involved in pathophysiological events associated with oxidative stress in cells and tissues. Therefore, some of these aldehydes are considered as secondary toxic messengers and disseminate oxidative stress to internal parts of cells [2] .
Based on their structural features, these aldehydes are mainly classified into three families: 2-alkenals, 4-hydroxy-2-alkenals, and ketoaldehydes [3] . 4-Hydroxy-2-alkenals such as 4-hydroxy-2-nonenal (HNE) and 4-hydroxy-2-hexenal are the most prominent aldehydes generated during peroxidation. HNE is highly stable and reactive [2] , and is largely responsible for many types of cellular damage associated with oxidative stress. HNE covalently binds to cysteine, lysine, or histidine residues [3] . These adducts are detected in several disease lesions, for example, in the brains of patients with the Alzheimer’s disease [4] [5] , and in low-density lipoproteins of atherosclerotic lesions [6] . HNE interferes with enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (EC 1.2.1.12) [7] .
The binding of HNE to proteins can cause conformational changes and amino acid modifications, as well as the accumulation and aggregation of proteins, which causes cellular dysfunctions. The breakdown of such modified proteins is an essential defense mechanism against oxidative stress in cells. The major proteolytic system for the degradation of oxidized or HNE-modified proteins is the ubiquitin-proteasome pathway [8] -[10] . While ubiquitin-dependent lysosomal degradation of HNE-modified proteins has been reported in human lens epithelial cells [11] .
GAPDH is a classical glycolytic enzyme that has a central role in energy production. Mammalian GAPDH displays a number of diverse activities unrelated to its glycolytic function. These include roles in membrane fusion, microtubule bundling, phosphotransferase activity, nuclear RNA export, DNA replication, and DNA repair. Moreover, GAPDH is involved in apoptosis, age-related neurodegenerative diseases, prostate cancer, and viral pathogenesis. Recent studies have also established its requirement for transcriptional control of histone gene expression, its necessity for the recognition of fraudulently incorporated nucleotides in DNA, and its mandatory participation in the maintenance of telomere structure [12] . Furthermore, GAPDH has an apparent role in Alzheimer’s disease-related apoptotic cell death [13] .
In previous reports, we indicated that GAPDH modified by acetylleucine chloromethyl ketone, a synthetic inhibitor of acylpeptide hydrolase, or HNE is degraded enzymatically and is accompanied by the release of a 23 kDa fragment in the U937 leukemia cell line [14] [15] . Furthermore, we purified the HNE-modified GAPDHdegrading enzyme from a U937 cell extract and rat neutrophils, and identified it as cathepsin G (EC 3.4.21.20) [16] [17] .
In the present study, we used U937 cells cultured in the presence of HNE to investigate the relation of proteasome and/or cathepsin G to GAPDH degradation.
2. Materials and Methods
2.1. Chemicals
Glyceraldehyde-3-phosphate (GAD) was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). SuccinylLeu-Leu-Val-Tyr-4-methylcoumaryl-7-amide (Suc-LLVY-MCA), t-butyloxycarbonyl-Leu-Ser-Thr-Arg-4-methylcoumaryl-7-amide (Boc-LSTR-MCA), benzyloxycarbonyl-Leu-Leu-Glu-4-methylcoumaryl-7-amide (Z-LLEMCA) and succinyl-Ala-Ala-Pro-Phe-4-methylcoumaryl-7-amide (Suc-AAPF-MCA) were purchased from the Peptide Institute (Osaka, Japan). HNE was from Cayman Chemical Co. (Ann Arbor, MI, USA). β-NAD was from Oriental Yeast (Tokyo, Japan). Other chemicals and solvents were of analytical-reagent grade.
2.2. Antibodies
A rabbit anti-GAPDH polyclonal antibody (pAb) was purchased from Santa Cruz Biotechnology Inc. (FL-335; sc-25778; Santa Cruz, CA). A rabbit anti-cathepsin G pAb was purchased from Calbiochem (219358). A biotinylated goat anti-rabbit immunoglobulin pAb (E0432), and horseradish peroxidase (HRP)-conjugated streptavidin (P0397) were obtained from Dako Denmark A/S (Glostrup, Denmark).
2.3. Cell Culture and Preparation of a U937 Cell Extract
The human leukemic cell line U937 was donated by the Cell Resource Center of the Biomedical Research Institute of Development, Aging, and Cancer (Tohoku University, Sendai, Japan) and cultured in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS, USA), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco BRL, Rockville, MD, USA) at 37˚C in a humidified atmosphere of 5% CO2 and 95% air and then subcultured every 3 days before the experiments were performed.
For other experiments, cells were plated in 6-cm diameter dishes (Falcon) at 5 × 105 cells/dish in 5 ml of culture medium and then cultured for 2 days at 37˚C. The medium was then replaced with FBS-free culture medium, and incubated at 37˚C for 2 h. Next, HNE (0, 5, 10, 20 and 40 μM) was added and the cells were incubated at 37˚C for a further 10 min. The culture medium was then supplemented with FBS to a concentration of 10% and the cells were cultured for 24 h.
To prepare the cell extract, U937 cells (3 × 106) were collected, washed twice in 0.5 ml of ice-cold Ca2+- and Mg2+-free phosphate-buffered saline (PBS) and treated with cell lysis buffer [20 mM phosphate buffer, pH 7.4, 1 mM EDTA, 0.05% (v/v) Triton X-]">100] for 3 min. The cell lysate was centrifuged at 10,000 × g for 5 min at 4˚C, and the resultant supernatant (U937 cell extract) was used for further analysis. The protein concentration was determined by the Bradford method [18] using bovine serum albumin as a reference standard.
2.4. Measurement of Activities of GAPDH in the U937 Cell Extract
GAPDH activity in the U937 cell extract obtained from HNE treatment was measured according to the procedure of Dimmeler et al. [19] with minor modifications [20] . A total of 80 μl of U937 cell extract was added to 125 μl of pre-warmed 50 mM triethanolamine buffer (pH 7.6) containing 50 mM arsenate, 2.4 mM glutathione, 0.4 mM β-NAD and 150 μg/ml GAP. GAPDH activity was determined at 37˚C for 1 min by measuring NADH production at 340 nm.
2.5. SDS-PAGE and Western Blotting
Protein samples were prepared in SDS-PAGE sample buffer [62.5 mM Tris-HCl, pH 6.8, 4% (w/v) SDS, 20% (v/v) glycerol, 10% (v/v) 2-mercaptoethanol, and 0.01% (w/v) bromophenol blue]. Aliquots of the samples were subjected to SDS-PAGE in 12% polyacrylamide gels according to the method of Laemmli [21] . For western blotting, gels were electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P Transfer Membrane; Millipore Co.) at 180 mA for 90 min using a semidry transfer system (Trans-Blot SD Cell; Bio-Rad Laboratories Inc., Hercules, CA, USA). The blotted membrane was blocked in 2% Block Ace Solution (Dainippon Sumitomo Pharma Co. Ltd,. Osaka, Japan) for 1 h, probed with the rabbit anti-GAPDH pAb (20 ng/ml) or rabbit anti-cathepsin G pAb (1 μg/ml) for 2 h, and then visualized using the biotinylated anti-rabbit immunoglobulin pAb, HRP-streptavidin, and Immobilon Western Chemiluminescent HRP Substrate (Millipore Co.). A LAS-1000 UV mini F85 Luminoimage Analyzer (Fujifilm Co., Tokyo, Japan) was used for chemiluminescence detection. The density of each band was quantified using Multi Gauge software (Version 3.0; Fujifilm Co.) and expressed in arbitrary units.
2.6. Assays for Enzyme Activities
Chymotrypsin-like, trypsin-like and peptidylglutamyl-peptide hydrolase activities of the proteasome were assayed using the fluorogenic substrates Suc-LLVY-MCA, Boc-LSTR-MCA and Z-LLE-MCA, respectively, according to the methods from previous reports [22] [23] . Cathepsin G activity was measured by a known method using Suc-AAPF-MCA as the substrate [24] . The fluorescence intensity of the solution was read at an emission wavelength of 460 nm and an excitation wavelength of 360 nm.
2.7. Statistical Analysis
One-way analysis of variance was carried out to determine the levels of significance in experiments. Multiple group comparisons were performed using the Student-Newman-Keuls test. Data are given as mean ± SE values.
3. Results and Discussion
HNE is an α,β-unsaturated aldehyde that is formed by the peroxidation of ω-6 polyunsaturated fatty acids. HNE is increased in hypercholesterolemia and in atherosclerotic lesions, causing accumulation of HNE-protein adducts [25] . In addition, HNE promotes endothelial oxidative stress [26] , endothelial barrier dysfunction [27] [28] , and apoptosis [29] . Recently, several publications report the role of HNE-protein adducts in the pathogenesis and progression of Alzheimer’s disease [30] -[32] . Since HNE-modified proteins cause many cellular dysfunctions, the removal of HNE-modified proteins has important consequences for cell survival. However, the degradation mechanisms of HNE-modified proteins have not yet been clarified. Previously, we found that GAPDH degradation is triggered by HNE and 4-hydroxy-2-hexenal and is catalyzed by an enzyme that differs from proteasomes and lysosomal enzymes [15] . Moreover, we purified the HNE-modified GAPDH-degrading enzyme from a U937 cell extract by dialysis and sequential chromatographic steps, and identified it as cathepsin G [16] .
In the present study, we examined whether GAPDH in U937 cells incubated with HNE in culture is degraded to a similar extent as that incubated with HNE and U937 cell extract. The concentration-dependent inhibition or degradation of GAPDH by HNE was examined at concentrations of 5 to 40 μM. The GAPDH activity of U937 cells was decreased by HNE treatment in a concentration-dependent manner (Figure 1(a)). In contrast, the density of the 36 kDa band corresponding to GAPDH was not decreased by HNE treatment at any of the concentrations examined (Figure 1(b)). The data suggest that GAPDH might be modified oxidatively by HNE resulting in a loss of activity, but oxidatively modified GAPDH is not degraded enzymatically and is not accompanied by the release of a 23 kDa fragment in the U937 cells.
 (a)
(a) (b)
(b)
Figure 1. Changes in GAPDH activity and GAPDH level in U937 cells treated with increasing concentrations of HNE. U937 cells were incubated with various concentrations of HNE for 10 min in culture. The cells were assayed for GAPDH activity (a), and for GAPDH level, which was analyzed by western blotting using anti-GAPDH pAb (b). Both the GAPDH activity and the GAPDH level are expressed as the percent of control. Data are mean ± SE (bars) values from five independent experiments. Significant decreases in GAPDH activity compared with control are indicated by asterisks: ***p < 0.001.
As the proteasome might contribute to the metabolism of oxidatively-modified proteins during oxidative stress in vivo, the concentration-dependent effects of HNE on proteasome activities were examined at concentrations of 5 to 40 μM using the fluorogenic peptides (Suc-LLVY-MCA, Boc-LSTR-MCA and Z-LLE-MCA) as proteolytic substrates. As shown in Figure 2(a), at the tested HNE concentrations, no effects on the proteasome activities (trypsin-like activity, chymotrypsin-like activity and peptidylglutamyl-peptide hydrolase activity) were observed. These data led to the assumption that HNE-induced oxidative stress did not affect proteasome activities.
In previous reports, we suggested that cathepsin G had an important role in eliminating HNE-modified proteins formed during exposure to oxidative stress. The concentration-dependent effect of HNE on cathepsin G activity and cathepsin G protein level was tested by culturing for 10 min at concentrations of 5 to 40 μM. Both the activity and the level of cathepsin G in the U937 cells were decreased by HNE treatment in a concentration-dependent manner. The decrease of the activity and the level of cathepsin G, which was about 45% compared with the control level, was observed at a concentration of 40 μM (Figure 2(b), Figure 2(c)). These results suggest
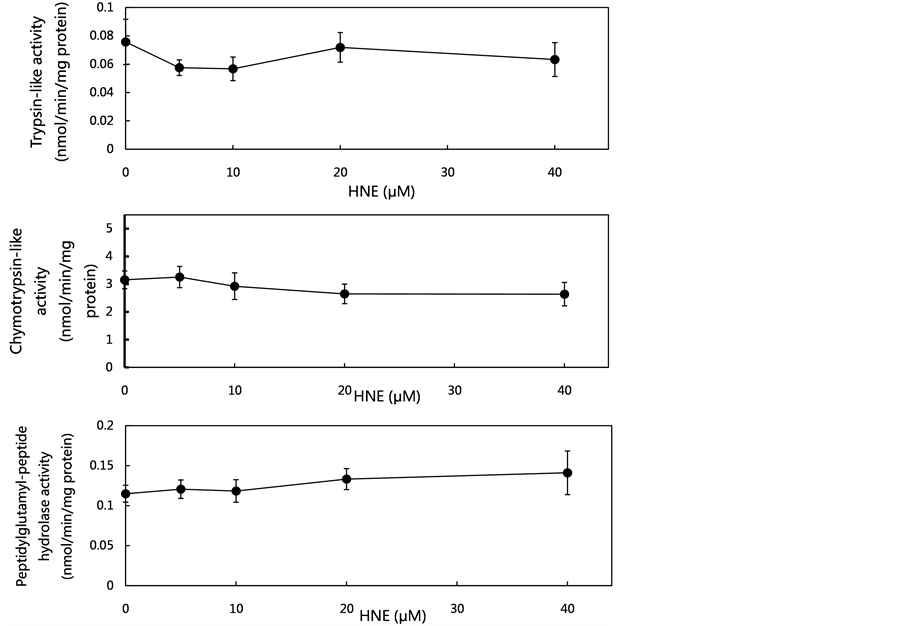 (a)
(a) (b)
(b) (c)
(c)
Figure 2. Effect of various concentrations of HNE on proteasome activities, cathepsin G activity and cathepsin G level in U937 cells. U937 cells were cultured in the presence of HNE for 10 min at concentrations of 5 to 40 μM. The proteasome activities in the U937 cells were measured using fluoropeptides, Boc-LSTR-MCA for the trypsin-like activity, Suc-LLVYMCA for the chymotrypsin-like activity, and Z-LLE-MCA for the peptidylglutamyl-peptide hydrolase activity (a). The cells were assayed for cathepsin G activity using Suc-AAPF-MCA as a substrate (b), and for cathepsin G level, which was analyzed by western blotting using anti-cathepsin G pAb (c). The cathepsin G level was expressed as the percent of control. Data are mean ± SE (bars) values from five independent experiments. Significant decreases in cathepsin G activity and cathepsin G level compared with control are indicated by asterisks: *p < 0.05, **p < 0.01, and ***p < 0.001.
that HNE-induced oxidative stress resulted in the decrease of cathepsin G activity, resulting in the loss of GAPDH degradation capacity in U937 cells.
It is of great interest to us that cathepsin G activity has a role in cell survival by eliminating HNE-modified proteins. We observed that addition of the specific cathepsin G inhibitor Z-Gly-Ler-Phe-chloromethyl ketone to U937 cells cultured with HNE significantly decreased the cell viability [16] . This result supports the proposal that cathepsin G activity is involved in promoting cell survival in the presence of HNE. Therefore, cathepsin G may serve to eliminate HNE-modified proteins in conjunction with proteasomes and lysosomal enzymes.
NOTES
*Corresponding author.