Comparative Study of Copper Adsorptivity and Selectivity toward Zeolites ()
1. Introduction
Heavy metals, such as Cu, Cd, Pb and Zn, are toxic to human beings and other living organisms if their concentrations exceed certain values. Copper is heavily used in industries for such uses as plating, mining and smelting, brass manufacture, electroplating, petroleum refining and Cu-based agrichemicals mining. The use of heavy metals in these industries produces a lot of wastewater and sludge containing Cu in various concentrations. These have negative effects on water and the environment in general [1] . Copper may also be found as a contaminant in various foods, especially in shellfish, liver, mushrooms and nuts [2] . It has been reported that excessive intake of Cu by humans may lead to severe mucosal irritation, hepatic and renal damage, widespread capillary damage and central nervous system problems [3] . The World Health Organization recommended a maximum acceptable concentration of Cu in drinking water of 1.5 mg·L−1 [4] . Therefore, the concentration of this metal must be reduced to a level that satisfies national and international environmental best practices (regulations) for various water bodies.
The most commonly used techniques for removing Cu ions from aqueous solutions include oxidation, reduction, precipitation, membrane filtration, biological process, ion exchange and adsorption. Adsorption of heavy metals on various materials has been conducted by researchers with the objective of finding efficient and cost effective means of removing heavy metals. Many researchers have been investigating low-cost adsorbents for heavy metal removal such as sawdust [3] , rice husks [5] , sago waste [6] , red pepper seeds [7] , treated Indian barks [8] , teak leaves powder [9] , saltbush leaves [10] , groundnut shells [11] , tree fern [12] , chitosan beads [13] and zeolites [14] [15] .
Zeolites, with their permanent negative charges as well as the interconnection of channels and cages that run through their secondary framework structure, are effective adsorbents for positively charged pollutants such as heavy metals [16] . Zeolites have a three-dimensional structure which constitutes of (Si, Al) O4 tetrahedra connected by all their oxygen vertices forming channels where exchangeable cations counterbalance the negative charge generated from isomorphous substitution. They have specific properties such as large surface area, high cation exchange capacity (CEC) and surface acidity so they are applied to many fields of industry as adsorbents, molecular sieves, ion exchangers and catalysts.
Zeolites can be found in nature (natural zeolites); they can be synthesized from chemical reagents (synthetic zeolites) or can be prepared from industrial by-products such as coal fly ash (artificial zeolites). In this study, zeolites A4, faujasite X, faujasite Y, Na-P1, clinoptilolite and modernite were used with the objective of removing Cu from an aqueous solution through adsorption and establishing a selectivity sequence. At the same time, in order to compare the selectivity between zeolites and other naturally existing minerals, montmorillonite was also used. Montmorillonite is a type of clay mineral that has been used as an adsorbent for a long time. It is a 2:1 type layer silicate mineral characterized by its interlayer space and external surface which are particularly suitable for reactions such as adsorption of heavy metal ions and organic compounds.
This study therefore aimed at finding the zeolite species that would give optimum adsorption results and describing the mechanism of adsorption for the particular zeolite. It also aimed at establishing a selectivity sequence of Cu toward these zeolites and montmorillonite. Although there are many zeolite species, not all of them are available and suitable for optimum adsorption of heavy metals such as Cu hence the need for research into which zeolite species can best optimize adsorption of Cu. Among the studies on Cu adsorption that have been conducted, the main focus has been on finding a single adsorbent that can adsorb Cu either from a mixture of heavy metals or a homogenous solution [17] - [20] . These studies have not focused on the use of various adsorbents for adsorption of a single heavy metal in order to determine selectivity among adsorbents hence the current study was conducted to obtain the adsorbent which has high selectivity toward Cu as well as high adsorption.
2. Materials and methods
2.1. Zeolite samples
The choice of zeolite samples for this experiment based on the variations in their CEC, the difference in their pore structures as well as their availability. Commercially available zeolite A4, faujasite X, faujasite Y, modernite, clinoptilolite and montmorillonite were used while Na-P1 was synthesized in this study. Zeolites A4 and faujasite X were supplied by Wako Pure Chemicals Industries Japan, faujasite Y was supplied by Tosoh Company, Japan, natural modernite from Fukushima Prefecture in Japan, natural clinoptilolite from Shimane Prefecture in Japan and natural montmorillonite was supplied by Japan Clay Science society. The above samples were used with Na+ saturation in order to have Na+ as the uniform exchangeable cation. Na-P1 was synthesized by adding a diluted solution of NaOH to a specified amount of NaAlO2. After a few minutes of stirring, NaSiO4 was added dropwise. This mixture was heated on a hot plate for 24 h and thereafter, the samples were washed 3 times with 100 mL water and dried at 40˚C for 24 h.
2.2. Sample characterization
Samples were characterized by powder X-ray diffraction (XRD) using a Rigaku Ultima IV X-ray Powder X-ray Diffractometer with Cu-Kα radiation generated at 40 kV and 40 mA, between 3˚ - 60˚ of 2θ angles with a sampling width of 0.02˚ and a scanning rate of 2˚ min−1. The amounts of Si and Al were measured by X-ray fluorescence (XRF) using a Rigaku RIX 2100 XRF. It was operated at a tube voltage of 30 kV and a tube current of 100 mA. Cation exchange capacity (CEC) was measured by initial saturation of samples with 1M KCl solution. The K+ saturated samples were washed with 1M NH4Cl solution in order to remove the exchangeable K+ ions. The amount of K+ in the supernatant was measured using atomic absorption spectrophotometer (AAS, Hitachi Z-5000) and the CEC was calculated.
2.3. Adsorption experiments
Adsorption of Cu was conducted using 50 mL of Cu(NO3)2 solution with initial concentrations of 0, 0.05, 0.10, 0.15, 0.20, 0.30, 0.45, 0.60 mM and 0.01 g of Na+ saturated zeolite and montmorillonite samples. The experiments were conducted in the presence of 100 mM NH4NO3 and a constant initial pH of 5: pH adjustment was done using 1 mM HNO3 by adding drops of HNO3 to a mixture of Cu(NO3)2 and NH4NO3 in concentrations that have been mentioned earlier. The coexistent NH4NO3 represents coexisting ions in the polluted waters. Experiments were conducted at room temperature using centrifuge bottles with aluminium foil covering in order to prevent precipitation due to sunlight. Shaking was done for 1 h which was experimentally pre-determined to be sufficient for adsorption equilibrium to be reached. After shaking, the samples were centrifuged at 1600 g for 10 min then pH of the supernatant was measured. Finally, the concentration of Cu in the supernatant was measured by AAS. The amount of adsorption of Cu was calculated from the difference between initial and final Cu concentrations.
3. Results and discussion
3.1. Characterisation of samples
The samples were analyzed before commencement of the adsorption experiments: CEC measurement, Si/Al ratio determination, and XRD analysis. Table 1 is an illustration of the CEC and Si/Al ratio of the samples. Zeolite A4 was the sample with the highest CEC of 6150 mmol·kg−1 followed by the rest of the samples.
Figure 1 shows the XRD patterns of the samples. The diffraction peaks for zeolites A4, faujasite X, faujasite Y and Na-P1 all correspond to the typical structures of the aforementioned samples that have been observed in literature [21] . Clinoptilolite and modernite both displayed patterns that correspond to those indicated by Dixon and Weed [22] . With montmorillonite, the XRD patters revealed the three main peaks of Na-montmorillonite. The pattern itself corresponded to that reported by Mering and Brindley [23] .
3.2. Copper adsorption isotherms
Preliminary experiments confirmed that adsorption equilibrium was reached within 1 h of shaking. At the same time, it was revealed through these experiments that an increase in pH to >6.5 resulted into the precipitation of
![]()
Table 1. CEC and Si/Al ratio of the samples.
![]()
Figure 1. RD patterns of the samples. a: A4, b: faujasite X, C: faujasite Y, d: Na-P1, e: clinoptilolite, f: modernite and g: mont- morillonite.
Cu hence experiments were conducted at an initial pH of 5 to avoid precipitation and to maintain the equilibrium pH to <6.5.
The results were analyzed using Langmuir and Freundlich isotherm analysis methods. The analysis of the isotherm data is important to develop an equation which accurately represents the results and which could be used for design purposes. There are essentially three stages in the adsorption process by porous adsorbents [24] : 1) solute transfer from the bulk solution to the external surface of the sorbent through a liquid boundary layer (film resistance); 2) solute transfer from the sorbent surface to the intra particle active sites (intra particle resistance); and 3) interactions of the solute with the available sites on both the external and internal surfaces of the sorbent (reaction resistance). One or more of the above-mentioned stages may control the rate at which the solute is adsorbed and the amount of solute that is adsorbed onto the sorbent. In this study, since various zeolites with different properties were used, most of the aforementioned factors may have played a role in the adsorptive selectivity of the samples. Figure 2 shows the adsorption isotherms of Cu on the previously mentioned samples.
The adsorptive capacity of the samples was analyzed using Langmuir analysis method. The Langmuir linear equation used was:
 (1)
(1)
where C is the equilibrium concentration (mmol·L−1), X the amount adsorbed (mmol·kg−1), Xm the maximum adsorption (mmol·kg−1) and K a constant related to binding energy (L·mmol−1). A plot of C/X against C resulted into a straight line for most of the samples with A4 having the highest correlation efficiency (R2) = 0.9943. The values of Xm, K and R2 determined by Langmuir analysis are tabulated in Table 2. The selectivity sequence thus determined was A4 > faujasite X > modernite > Na-P1 ≈ montmorillonite ≈ faujasite Y > clinoptilolite. The Freundlich linear equation was also used for analyzing the adsorption data. The linear equation used was:
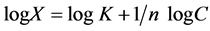 (2)
(2)
where X = amount of substance adsorbed (mmol·kg−1), C = Equilibrium concentration (mmol·L−1), K = constant related to both the strength and amount of adsorption while 1/n = the intensity of adsorption. The magnitude of the exponent 1/n gives an indication of the favorability of adsorption. Values of n, where n > 1 represent favorable adsorption condition. The values of K, n and R2 calculated by the linear form of Freundlich model are illustrated in Table 2. Zeolite A4 had the highest K and n values as was the case with Langmuir analysis.
In general, Cu had the highest selectivity toward zeolite A4 which had the highest adsorptive capacity. The adsorption isotherm for A4 was very steep in the lower concentration range compared to the other samples. This
![]()
Figure 2. Copper adsorption isotherms on zeolites A4, faujasite X, modernite, Na-P1, montmorillonite, faujasite Y and clinopti- lolite. 0.01 g of each sample were mixed with 50 mL of 0 - 1.2 mmol·L−1 CuNO3 in the presence of 100 mM NH4NO3, and shaken for 1h. Initial pH was 5 for all experiments.
![]()
Table 2. Langmuir and Freundlich parameters of Cu adsorption.
resulted in A4 having the highest Langmuir K constant compared to all other samples. The steep nature of the isotherm further indicated that Cu was more strongly attached to zeolite A4 than the rest of the samples, indicating that A4 would be used efficiently for the adsorption of Cu in nature at low concentrations. This was followed by faujasite X and the rest of the samples. On the contrary, clinoptilolite which had the lowest Xm also had an isotherm that was almost flat in the lower concentration range, implying low adsorption and a weaker binding strength of Cu to the adsorbent. The Langmuir K constant which relates to binding energy of the samples however did not exactly follow a similar order to that of adsorption. This meant that although Cu was highly adsorbed onto some samples in terms of quantity, the bond that was formed between Cu and available sites of the adsorbent was not very strong.
Modernite was one of the samples whose adsorptive capacity was comparatively lower than all the synthetic zeolites but the Langmuir K constant was high, indicating that Cu was strongly adhered to this sample. In other words, the force of attraction between the adsorbent and adsorbate was very strong even though the amount of adsorption was low.
In a related study, Hui et al. [25] experimented on the adsorption and selectivity sequence of mixed metal ions Co2+, Cr3+, Cu2+, Zn2+ and Ni2+ toward zeolite A4 in a batch experiment system. Results indicated a selectivity sequence of Cu2+ > Cr3+ > Zn2+ > Co2+ > Ni2+. The amount of Cu adsorption on A4 however was around 794 mmol·kg−1, which is almost half of the amount of adsorption of 1429 mmol·kg−1 in current experiment. Factors such as crystal structure of zeolite 4A, free energy of hydration and hydrated radii of the metal ions as well as the CEC of the adsorbent may be responsible for the observed selectivity in this study. Zeolites, in general, are weakly acidic in nature and therefore sodium form exchangers are selective for protons (RNa + H2O ⇔ RH + Na+ + OH−). This leads to high pH values when the exchanger is equilibrated with a relatively dilute electrolyte solution [26] , making feasible the metal hydroxide precipitation. The crystal structure of zeolite 4A contains large cages having a near spherical shape and free diameter of 11.4 Å. Each of these cages is connected with six neighboring cages via eight-membered rings (8-MR) having a crystallographic diameter of 4.1 Å. The effective pore width of zeolite 4A is 4 Å. A common factor preventing a group of metal ions from being adsorbed by zeolite 4A is the size of the hydrated ion. If the hydrated ion size is greater than that of the pore, the species may be excluded or some of the waters of hydration must be stripped from the solvated ions to enable them to enter the pores of the zeolite. Copper has a hydrated radius of 4.19Å [27] an unhydrated radius of 0.82 Å and a free energy of hydration of −498.7 kcal g−1-ion [25] . Although the hydration radius was as such, there is high possibility that some amount of Cu penetrated inside the pore spaces of zeolite A4.
In related studies montmorillonite in Na form adsorbed 47.8 mmol·kg−1 of Cu [17] . This adsorptive capacity was lower than that of Na montmorillonite in the current experiment which was 116 mmol·kg−1. This was mainly because the experiment by Abollino et al. [17] was conducted at varying pH range of 2.5 - 8.0 and in the presence of ligands, forming complexes of different stabilities with the metals of interest since the study included other metals like Cd, Cr, Cu, Mn, Ni, Pb and Zn. The pH variations influenced to a higher extent the concentrations of Cu, Pb and Cd in the effluent used in the experiment. Moreover the results suggested that complex formation hindered the adsorption of the metals on the clay, hence the lower adsorptive capacity than the current study. Clinoptilolite and Fe modified clinoptilolite on the other hand had adsorptive capacities of 214 mmol·kg−1 and 590 mmol·kg−1 [28] for Cu which was higher than the 74 mmol·kg−1 recorded in current study. The main factors that contributed to different adsorption capacities of the two solids (clinoptilolite and Fe modified clinoptilolite) were the new surface species and negative charge of the clinoptilolite-Fe system. In addition, it was found that for most of the samples the clinoptilolite-Fe system released lower concentrations of counterbalance cations (Ca, Mg and Na) and higher concentrations of K than clinoptilolite, while the dissolution of Si/Al was limited. These factors coupled with the difference in the solid: solution ratio may have contributed to the difference in the adsorptive capacities of this experiment and the experiment reported in this paper.
Faujasite X, faujasite Y, N a-P1, clinoptilolite and modernite on the other hand have pore sizes of 7.4 Å [27] , 7.3 Å & 4.8 Å [29] , 4.5 - 6 Å [30] and 4.2 Å [31] , respectively. According to the effective pore sizes of zeolites A, X and Y, the amount of metal ions adsorbed on zeolite X and Y should exhibit more than zeolite A. However, the effective pore size of zeolites A, X and Y do not explain clearly, the high adsorption capacity of the synthetic zeolite A. It is therefore thought that the main mechanism involved in the sorption of Cu in this case is based on cation exchange capacity of zeolites or its charge density. It was observed that the order of selectivity of adsorption was closely linked to the cation exchange capacities of the samples. The sample with the highest CEC also had the highest adsorptive capacity and vice versa. The difference in the amounts of Cu adsorption between faujasite X and faujasite Y which have the same frame structure also indicated that the CEC is was one of the key factors influencing Cu adsorption.
3.3. Analysis of exchangeable cations released at equilibrium
An analysis of Na, Si and Al released after the adsorption experiment was conducted. The amount of Na released has been expressed in mmol·kg−1 of charge. However, the amount of adsorption expressed in previous explanations was in mmol·kg−1 of Cu. Being a divalent ion, the amount of adsorption expressed herein needs to be expressed in moles of positive charge in order to compare with the CEC values. The amount of Cu adsorption in this study was much lower than the CEC of all the samples. This was thought to be due to the availability of other sources of positive charge which replaced Na on the surface of the adsorbent. The sources of positive charge were the addition of H+ from HNO3 which was used for pH adjustment, NH4+ as a background solution, and also charge from hydroxy-aluminium cations which were released from the zeolite structure. After the adsorption experiment it was noted that there was some amount of Si and Al released into the supernatant. Altogether, the experiment medium had Cu, H+, NH4+, and hydroxy-aluminium cations as sources of positive charge which were available for an exchange with Na present on the surface of the adsorbents hence the high release of Na at equilibrium in comparison to the amount of Cu adsorption.
Zeolite A4, faujasite X and Na-P1 released Na in quantities that were within the range of the CEC of these samples (CEC values are indicated in Table 1). The amount of Na released for A4, faujasite X, faujasite Y, Na-P1, modernite, montmorillonite and clinoptilolite at the highest initial Cu concentration was: 6479, 5389, 1742, 4856, 1495, 436 and 1232 mmol·kg−1 respectively. Zeolites A4 and faujasite were noted to have higher proton selectivity because these samples adsorbed protons, releasing Na into the supernatant even when Cu adsorption was at its lowest. Additionally, they can adsorb protons even at pH of 6 to7 according to experiments conducted earlier whose results are not shown in this paper. An analysis of the amount of Na released and the amount of Cu adsorbed revealed that some amount of Na was released even with very low adsorption of Cu. This was largely due to the available protons in the experiment medium especially 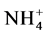 which was added in the highest volume and concentration. Figure 3 is an illustration of this phenomenon. The intercepts in the figure are the amounts of Na replaced by
which was added in the highest volume and concentration. Figure 3 is an illustration of this phenomenon. The intercepts in the figure are the amounts of Na replaced by 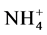 at the blank runs. The ratio, (Na replaced by NH4)/CEC, is related to Na-NH4+ exchange selectivity of each sample. A high ratio means high selectivity of the sample toward
at the blank runs. The ratio, (Na replaced by NH4)/CEC, is related to Na-NH4+ exchange selectivity of each sample. A high ratio means high selectivity of the sample toward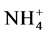 .
.
The figure further indicates that for A4 and Faujasite X, the amount of Na released increased with an increase in adsorption (almost all the available Na was released) while for the rest of the samples, the release of Na decreased with an increase in adsorption (about half of the available Na was released). For A4, faujasite X and Na-P1, almost all available Na is released into the solution while for the rest of the samples; about half of the available Na is released. There was an almost 1:1 relationship between the amount of Na released and the amount of Cu adsorbed for A4, faujasite X and Na-P1. Since among the samples, proton selectivity is higher for A4, followed by Faujasite X, H+ as well as 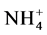 were strongly attached to these samples and the weaker Na was released into solution hence the high release of Na. A study by McBride [32] on Cu interaction with kaolinite reported a similar observation. The study reported that most of the exchangeable Na was released to solution by proton (H+) exchange even before Cu became significant in the exchange process, implying that even before adsorption of Cu had significantly commenced, Na was present in the supernatant.
were strongly attached to these samples and the weaker Na was released into solution hence the high release of Na. A study by McBride [32] on Cu interaction with kaolinite reported a similar observation. The study reported that most of the exchangeable Na was released to solution by proton (H+) exchange even before Cu became significant in the exchange process, implying that even before adsorption of Cu had significantly commenced, Na was present in the supernatant.
Although the release of Na was largely influenced by NH4+, the effect of H+ though comparatively minimal was further observed from the change in pH: an increase in pH of 0.301 indicates adsorption of half of H, from the definition of pH. This implies that for A4 and faujasite X with greater proton selectivity, nearly 100 % of the proton was adsorbed hence the slightly high rise in pH compared to the rest of the samples.
On the other hand, it is known that the Si/Al molar ratio of zeolite A (=1.00) is lower than that of zeolite X (=1.24). The smaller Si/Al molar ratio in the zeolite framework produces more charge deficiency. Thus, the framework needs more Na to compensate the excess negative charge and more exchangeable Na exist in the zeolite structure. In other words, the amount of exchangeable Na is inversely proportional to the Si/Al molar ratio in the framework of the zeolite structure [19] . This fact also explains the reason behind the high release of Na from the samples with high CEC and high adsorptive capacity in the current study. A similar observation was reported in a study by Breck on the adsorption of Cu on zeolite A and X [33] . Thus, the amount of exchangeable Na (or proton since at equilibrium, more H+ than Na existed on the surface of the samples especially A4, Fauja-
![]()
Figure 3. Amount of Na released and the amount of Cu ad- sorbed for zeolites A4, faujasite X, faujasite Y, Na-P1, mo- dernite, montmorillonite and clinoptilolite.
site X and Na-P1) bonded to zeolites framework plays an important role in the removal performance of heavy metal ions.
With faujasite Y, modernite and clinoptilolite and montmorillonite, the amounts of Na released were below the CEC. This was due to the low selectivity of these samples for Cu and the other available protons which led to a lower adsorptive capacity as well. This meant that not all the Na available within the samples could be replaced by the Cu or other protons. In other studies, especially those which were conducted at high pH of ≥6, it was considered that the mechanism of Cu removal was based on precipitation of metal hydroxides on the surface of zeolites or inside the pore walls. In the study on heavy metal adsorption on natural zeolite (clinoptilolite) by Filippidis [34] , a selectivity sequence of Fe3+ > Zn2+ > Cu2+ > Mn2+ was reported. This sequence however, was not according to the hydration diameters of the heavy metals. The hydration diameters of the samples would give a selectivity sequence of Cu2+ > Zn2+ > Mn2+ > Fe3+ because the hydration radius is smallest for Cu and highest for Fe3+. It was further reported that the difference in the selectivity series may be an indicator that adsorption is not necessarily the only mechanism responsible for the removal of heavy metal ions from solution; precipitation of the metal hydroxides may have a significant influence in the treatment process by natural zeolite.
Results also showed the presence of Si and Al in the supernatant for some of the samples. The leaching of Si- and Al-species may be due to the effect of the hydrogen ions on the aluminosilicate framework of the zeolite, which initially causes a rupture of the Al-O bonds and further detachment of Al- and Si-species through hydrolysis reactions [35] . It was however observed that the release of hydroxy-aluminium was higher for all samples compared to that of Si with clinoptilolite and modernite releasing neither Si nor hydroxy-aluminium. Table 3 is a summary of the amount of Si and Al released from the sample with the highest initial concentration of Cu. The release of Al was influenced by the fact that the H+ present in the experiment medium initially attacks the negatively charged oxygen atom of the tetrahedral Al atom, the O-A1 linkage is ruptured and the A1 atom undergoes some structural rearrangements. The detachment of the A1 atom is a consequence of successive ruptures of the remaining A1-O bonds after a proton attack on the oxygen atom [36] .
3.4. Assessment of the equilibrium pH
The pH of the aqueous solution is an important controlling parameter in the sorption process [37] and metal removal typically increases with increasing pH values [38] . The pH may affect the ionization degree of the sorbate and the surface property of the sorbent [39] . Chemically, the solution pH influences metal speciation. For instance, heavy metal ions may form complexes with inorganic ligands such as OH−. The extent of the complex formation varies with pH, the ionic composition and the particular metal concerned. The exact speciation of a metal has a significant impact on the removal efficiency of the zeolites. The selectivity of metal ion by zeolites is also influenced by the character of the metal complex that predominates at a particular solution pH. Exposure of the zeolite surface to water causes the ionization of surface hydroxyl groups (Si OH and Al OH). The degree of ionization depends on pH, and the acid/base reaction occurring at the hydroxyl groups may results in surface charge development [40] .
At low pH, the number of available hydrogen ions is high and Cu ions have to compete with them for the adsorption sites on the adsorbent surface, leading to lower adsorptive capacity. As pH gradually increases to around 5 or 6, adsorptive capacity is enhanced due to the reduction in the amount of H+ available which leads to less competition for the available sites of adsorption. At the initial pH of 5 in this experiment, it was considered that this was the optimum pH at which adsorption could be possible without much influence from H+ as well as controlled precipitation of Cu. The active sites on the adsorbent surface are weakly acidic in nature and with increase in pH, they are gradually deprotonated favoring more and more Cu uptake. Similar adsorption mechanism has been reported by various authors [41] - [43] . Table 4 is a summary of the equilibrium pH that was recorded for the samples. Although the initial pH in current experiments was maintained at 5, there was variability in the equilibrium pH with A4 having a comparatively higher equilibrium pH. There was a slight rise in equilibrium pH from the initial pH of 5 for the samples. Although A4 and faujasite had a rise in pH to a value that was slightly higher than 6 (6.22 being the highest for A4 and 6.19 the highest for faujasite X), this rise in pH was not considered to have affected the adsorption process. Preliminary experiments which were conducted prior to this experiment confirmed that adsorption was constant between pH 5 - 6.5.
![]()
Table 3. Amounts of Si and Al released at highest initial concentration of 0.6 mM.
![]()
Table 4. Changes in the equilibrium pH after 1 h of shaking with an initial pH of 5.
4. Conclusion
The adsorptive selectivity of Cu on various zeolites and montmorillonite has been studied. From the results of this experiment, it can be concluded Cu has the highest selectivity toward synthetic zeolites, A4 to be specific, followed by artificial zeolites, then natural zeolites. The selectivity sequence so derived was A4 > faujasite X > modernite > Na-P1 ≈ montmorillonite ≈ faujasite Y > clinoptilolite. Further to that, the adsorption of Cu toward zeolites is mostly influenced by the CEC of the zeolite sample in question. The hydration status of Cu also has the potential of influencing its adsorptivity though CEC plays a much greater role. In some cases, like the case with natural zeolites, precipitation of Cu also possibly influenced their adsorptive capabilities. The results from this study would be useful in deciding a clean-up option for waste water polluted by heavy metals especially in highly industrialized nations where heavy metal pollution is an issue of serious concern.
NOTES
*Corresponding author.