Antimicrobial susceptibility of strains of Enterobacteriaceae isolated from bloodstream infections using current CLSI and EUCAST breakpoints ()
KEYWORDS
Antimicrobial-Susceptibility; CLSI Breakpoints; EUCAST Breakpoints; Enterobacteriaceae; Bloodstream-Infections
1. INTRODUCTION
Bloodstream infections (BSI) are a leading cause of morbidity and mortality. In addition there is an emergence of extended spectrum β-lactamase (ESβL) producers along with an alarming increase and spread of multidrug-resistance among BSI pathogens [1-6]. Antimicrobial resistance surveillance of the local epidemiology is indispensable for the initial antibiotic therapy that in bloodstream infections is always empirical [7].
From 2010 the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [8] and the Clinical and Laboratory Standard Institute (CLSI) [9] published lower susceptibility breakpoints for third-generation cephalosporins to differentiate ESβL-positive from ESβLnegative isolates of Enterobacteriaceae. Moreover, breakpoint values of other classes of antibiotics mainly for Gram-negative species, were also reviewed [8,9]. Changed CLSI guidelines or the use of EUCAST guidelines have led differences in susceptibility rates, mainly for cefalosporins, and conflicting results in literature [10,11].
The aim of the present study was to compare the antimicrobial susceptibility results to different classes of antibiotics of Enterobacteriaceae isolates from bloodstream using 2013 CLSI [12] and 2013 EUCAST [13] guidelines to evaluate the impact of breakpoint discrepancies on the hospital policies and to give useful information to clinicians to elaborate correct guidelines in initial empirical therapy of bloodstream infections.
2. EXPERIMENTAL SECTION
Bacteria and Susceptibility Testing
We analyzed the MIC results of 512 strains of Enterobacteriaceae (232 E. coli, 224 K. pneumoniae, 40 Enterobacter spp and 16 Proteus spp) isolated from blood specimens from January 2009 to March 2013. Cultures were performed at the central Laboratory of Analysis of the Department of Bio-Medical Sciences of the University of Catania using the BD BACTEC ™ 9000 System (Becton Dickinson) with fluorescence detection technology and identification and antimicrobial susceptibility of the clinical isolates were determined using BD Phoenix™. For epidemiological purpose the isolates collected were re-identified using API 20 E (Oxoid) for Enterobacteriaceae and confirmatory testing was carried out by broth microdilution using CLSI methodology [12]. Only one isolate was included for each bacteraemic episode. ESβL and carbapenemase production was detected by screening and confirmatory tests suggested by CLSI guidelines [12]. The antibiotics tested for Gram-negative isolates included ampicillin, amoxicillin/clavulanic acid, piperacillin, piperacillin-tazobactam, imipenem, meropenem, aztreonam, cefepime, cefotaxime, ceftazidime, amikacin, gentamicin, ciprofloxacin and levofloxacin. Quality control testing was performed following CLSI guidelines [12]. For this retrospective study the MIC results were interpreted following the interpretive breakpoints published in 2013 by CLSI [12] and by EUCAST [13].
Percentages obtained by CLSI and EUCAST methodologies were compared using the chi-square test.
3. RESULTS AND DISCUSSION
3.1. Results
Table 1 shows the MIC range, MIC90 and antimicrobial susceptibility data of 512 Enterobacteriaceae isolates as classified by CLSI 2013 [12] and EUCAST 2013 [13] interpretative breakpoints.
ESβL-positive isolates were 200/512 (39%).
For amoxicillin/clavulanic acid and ampicillin EUCAST includes, in the resistant category, the MIC values classified as intermediate by CLSI, but different percentage of resistance was only observed for amoxicillin/clavulanic acid (70% and 64% respectively). For piperacillin alone or with tazobactam, CLSI and EUCAST susceptible breakpoints are ≤ 16 mg/l and ≤ 8 mg/l and resistant breakpoints ≥ 128 mg/l and ≥ 32 mg/l respectively. Therefore higher intermediate or resistant rates were observed using EUCAST breakpoints. Significant differences were observed between CLSI and EUCAST results for amoxicillin/clavulanic, piperacillin and piperacillin/tazobactam (p values for comparison < 0.0001, = 0.001 and < 0.0001, respectively). Even if imipenem and meropenem CLSI susceptible breakpoints are more restrictive than those of EUCAST (≤ 1 mg/l and ≤ 2 mg/l respectively), similar percentages of susceptibility were observed for both carbapenems; instead discrepancies were found for intermediate and resistant rates. In our study MHT performed for intermediate or resistant isolates to both carbapenems using different interpretive criteria, confirmed that only the current CLSI and not EUCAST breakpoints were able to detect 8% of the isolates producing carbapenemase (MIC ≥ 4 mg/l). Aztreonam susceptibility and resistance breakpoints respectively are ≤ 4 mg/l and ≥ 16 mg/l for CLSI and ≤ 1 mg/l and ≥ 8 mg/l for EUCAST. Results show that irrespective of the breakpoints used, 100% of all the ESβL-negative isolates were susceptible (MIC ≤ 1 mg/l) and 100% of the ESβL-positive isolates were resistant (MIC ≥ 32 mg/l) to this agent. Susceptibility breakpoints according to CLSI are ≤ 8 mg/l for cefepime, ≤ 1 mg/l for cefotaxime and ≤ 4 mg/l for ceftazidime; susceptibility EUCAST breakpoint is ≤ 1 mg/l for the three cephalosporins. The remarkable discrepancy between the two sets of recommendations for cefepime determined a shifting of ESβLnegative strains with MIC of 2 - 4 mg/l from susceptible to intermediate category and a significant difference between CLSI and EUCAST results (p < 0.0001). Instead, irrespectively of the breakpoints used, 100% of ESβL negative isolates was susceptible to cefotaxime and ceftazidime.
For amikacin and gentamicin, EUCAST breakpoints for susceptible, intermediate and resistant categories are one dilution lower than CLSI breakpoints, therefore using EUCAST criteria percentages of susceptible strains to these aminoglycosides were lower than those obtained using CLSI (90% vs 100% for amikacin, 66% vs 72% for gentamicin). Differences between CLSI and EUCAST results were statistically significant (p < 0.0001).
For ciprofloxacin and levofloxacin EUCAST breakpoints for the susceptible, intermediate and resistant categories are one dilution lower than those suggested by CLSI but no significant difference was determined.
Discrepancies in MICs using BD Phoenix and broth microdilution were not observed.
3.2. Discussion
An updated knowledge of the local epidemiology of antimicrobial resistance based on susceptibility testing is necessary when selecting antibiotics for formulary inclusion and for the initial empirical antibiotic-therapy of bloodstream infections [1]. The determination of susceptibility is dependent on the breakpoints used that vary somewhat based on the agency. Because CLSI 2013 [12] and EUCAST 2013 [13] still suggest different breakpoints, discrepancies due to the guidelines adopted by clinicians could lead to an important impact on the selection of the first-line antibiotic to be used in bloodstream infections increasing the use the carbapenems and loading to resistance and loss of therapeutic treatment options [10,11,14].
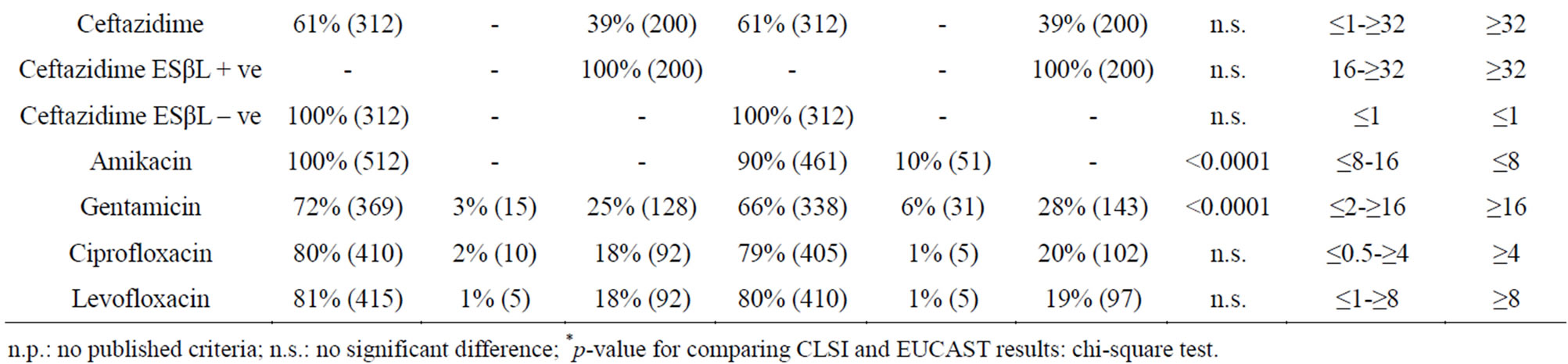

Table 1. Antimicrobial susceptibility of 512 strains of Enterobacteriaceae isolated from bloodstream infections as classified by CLSI 2013 [12] and EUCAST 2013 breakpoint criteria [13].
n.p.: no published criteria; n.s.: no significant difference; *p-value for comparing CLSI and EUCAST results: chi-square test.
Discrepancies between CLSI and EUCAST breakpoints for extended-spectrum cephalosporins have a significant impact on whether an invasive ESβL-producing isolate is classified as susceptible to these agents. Schito et al. [15] demonstrated that, in general, discrepancies between CLSI 2009 and EUCAST resulted in modest (≤ 4%) differences in the percentages of susceptible isolates of E. coli, responsible for UTIs, for all antimicrobial agents tested with the exception of cefuroxime (95% vs 82%), but the study did not screen isolates for ESβL production. Hombach et al. [16] have demonstrated that significant differences in the susceptibility rates of important cephalosporins such as cefepime, ceftazidime and cefotaxime applying EUCAST 2013 and CLSI 2013 guidelines were detected for ESβLand AmpC β-lactamase-producing isolates. Using CLSI 2010 or EUCAST breakpoints, Hawser et al. [17] and Kristo el al. [18] demonstrated that a proportion of ESβL-positive isolates may be reported as susceptible to expandedspectrum cephalosporins, leading to possible infection control and therapeutic implication. Moreover Hawser et al. [17] suggested that confirmation testing of ESβL phenotypes to ceftazidime, ceftriaxone, and cefotaximecould be helpful to monitor evolving epidemiology of ESβL-positive isolates. In this retrospective study, on the basis of MIC results of aztreonam and cephalosporins tested for Enterobacteriaceae, using 2013 CLSI [12] or EUCAST breakpoints [13], it was possible to differentiate ESβL-positive (MIC ≥ 4 mg/l) from ESβL-negative isolates (MIC ≤ 1 mg/l) for aztreonam, cefotaxime and ceftazidime. The ESβL-negative strains classified as intermediate to cefepime according to EUCAST and included in the susceptible category by the CLSI, suggest that less restrictive CLSI breakpoints for this cephalosporin could better designate the ESβL-negative isolates. However discrepancies between studies might be attributable to differences in regional prevalence of the ESβL type in E. coli, as suggested by Rodriguez-Baño et al. [19].
Using CLSI 2010 and or EUCAST 2011 guidelines, discrepancies with piperacillin-tazobactam were found by Rodriguez-Baño et al. [19] in the percentages of susceptibility to piperacillin-tazobactam, particularly among CTX-M1 producers. In our study, using current CLSI or EUCAST guidelines discrepancies were statistically significant for amoxicillin/clavulanic acid, piperacillin alone or with tazobactam.
In our study, significant differences (p < 0.0001) were found in the results using CLSI or EUCAST methodologies for imipenem and carbapenem. However eight percent of the strains with Modified Hodge Test (MHT) positive results can be discarded only using CLSI breakpoints.
Adopting current CLSI and EUCAST breakpoints significant discrepancies (p < 0.0001) were found for amikacin and, at less extent, for gentamicin. Statistically significant discrepancies in the susceptibility (p < 0.01) have been also found for amikacin by Rodriguez-Baño et al. [19].
The adoption of more restrictive EUCAST breakpoints, that are one dilution lower than those suggested by the CLSI, could permit a rapid detection of plasmid-mediated resistance to fluoroquinolones, that causes only a modest increase in MICs [15].
On the basis of this study, the adoption by clinical laboratories of current CLSI or EUCAST interpretive criteria, for these antimicrobial agents, could influence the decision to be taken by the physicians managing patients with bloodstream infections caused by Enterobacteriaceae and determine treatment implications. Anyway more clinical data are necessary to support the present CLSI and EUCAST criteria in different infections. Because differences in susceptibility rates are still detected, further harmonization of CLSI and EUCAST breakpoints is warranted.
ACKNOWLEDGEMENTS
We are grateful to Dr. Filippo Palermo of the University of Catania for statistical support.