Synthesis of Multifunctionalised 2-Substituted Benzimidazoles Using Copper (II) Hydroxide as Efficient Solid Catalyst ()
1. Introduction
Among the various nitrogen containing heterocycles, benzimidazole derivatives exhibit antiviral, antiulcer, antihypertensive and anticancer properties [1-4]. The benzimidazoles are biologically potent [5-7] and this moiety is an important pharmacophore [8,9] in drug discovery and also a good intermediate [10,11] for synthesis of many important organic compounds. Generally, the synthesis of 2-substituted benzimidazoles involves the treatment of o-phenylenediamines with either carboxylic acids [12-16] or their derivatives (nitriles, imidates or orthoesters) [17-19], under strongly acidic conditions and sometimes combines with very high temperatures (i.e., PPA, 180˚C) or the use of microwave irradiation [20-22]. These derivatives also often generated from the condensation of o-phenylenediamines with aldehydes [23,24] under oxidative conditions [25-31] using various oxidative and catalytic reagents [32,33] such as nitrobenzene (high-boiling point oxidant/solvent) [32,33], 1,4-benzoquinone, PhI(OAc)2, Zn-proline, heteropoly acids, thionyl chloride-treatment, 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ), electron-deficient olefins, benzofuroxan, MnO2, Pb(OAc)4 [26], oxone, NaHSO3, H2O2/HCl, Iodine & hypervalent iodine (iodobenzene diacetate (IBD), Na2S2O5, air, sulfamic acid, FeCl3·6H2O, In(OTf)3, Yb(OTf)3, Sc(OTf)3, Cu(OTf)2, KHSO4, IL, (bromo dimethyl) sulfoniumbromide, p-TSA, ZrOCl2∙8H2O, ZrCl4, HfCl4, borontrifluoride etherate, copper complex, polyaniline salt, Heuland natural zeolite, NaYzeolite, polymer-supported hypervalent iodine, TsOH/graphite and N,N-dimethyl aniline/graphite, cobalt(III) salen complex on activated carbon, cobalt(II) chloride hexahydrate, VO(acac)2- CeCl3 combo catalyst, gold/CeO2, AlKIT-5 copper iodide catalysts have been employed as the reagents or catalysts [32,33] for the synthesis of benzimidazoles. Although the reaction was efficiently promoted by the above conditions, they are often homogeneous catalysts and some of these methods suffer from one or more disadvantages such as usage of stoichiometric or more quantity of reagent, high cost of the catalysts, prolonged reaction times, occurrence of several side reactions, severe reaction conditions, difficulty in separation of the products from the reaction mixture and strong oxidizing nature of the reagents. Therefore, the discovery of mild and practicable, stable, cheap, recyclable, and ecofriendly heterogeneous catalysts for synthesis of 2-substituted benzimidazoles continues to attract the attention of researchers.
Recently the uses of heterogeneous catalysts [34-45] including zeolites [32,33,36-42] and nanoporous materials [43-45] have been received considerable importance in organic synthesis because of their ease of handling, enhanced reaction rates, greater selectivity, and simple workup. Since we have published the preparation of nanocrystals of cobalt oxides and hydroxides [46], we have sought the application as catalysts for organic reactions. Along this process, we have found that Cu(OH)2s which are as purchased solid catalysts exhibit catalytic activity for benzimidazole derivative synthesis. These solid catalysts are commercially available, non-hazardous, clean, cost-effective compared with other heterogeneous catalysts. In the continuation of our interest on catalytic applications of various heterogeneous catalysts, herein we report for the first time a simple, convenient and efficient method for the synthesis of multi-functionalised benzimidazole and its derivatives using Cu(OH)2 as a reusable solid catalyst. Copper (II) hydroxide is a pale blue, gelatinous solid and rarely found as an uncombined mineral, because it slowly reacts at room temperature in methanol using Cu(OH)2s as reusable solid catalysts. However, to the best of our knowledge, there has been no report available on the synthesis of multi-functionalised benzimidazoles using Cu(OH)2 in the open literature so far.
2. Results and Discussion
Initially, we have attempted the condensation of o-phenylenediamine 1 (1.0 mmol) with benzaldehyde 2 (R = Ph) (1.2 mmol) at room temperature in methanol condition for 6h at open oxygen atmosphere, in absence of catalyst by M.A.Chari et al., [32,33] and they have reported 27.6% of 2-phenyl benzimidazole product 3 and 0.1% of 1-benzyl-2-phenyl-1H-benzimidazoleproduct 4 at 6 h and on long reaction time (12 h) resulted the formation of a mixtures like 2-phenyl benzimidazole 3 (29.1%) and 1-benzyl-2-phenyl-1H-benzimidazole 4 (2%) as side-product. The detailed mechanistic study on oxygen-catalyzed coupling of o-phenylenediamine and benzaldehyde was carried out by Smith and Ho [47]. We have performed control experiments in absence of air under N2 atmosphere using Cu(OH)2 catalyst, but the reaction was not proceeding much, these gave very low yields of 3 (7%, 9%, 17%) at 6 h at RT to optimise the conditions. This indicates the presence of oxygen is important for the reaction. We have carried out the reaction using different amounts of catalyst (Table 1). It was
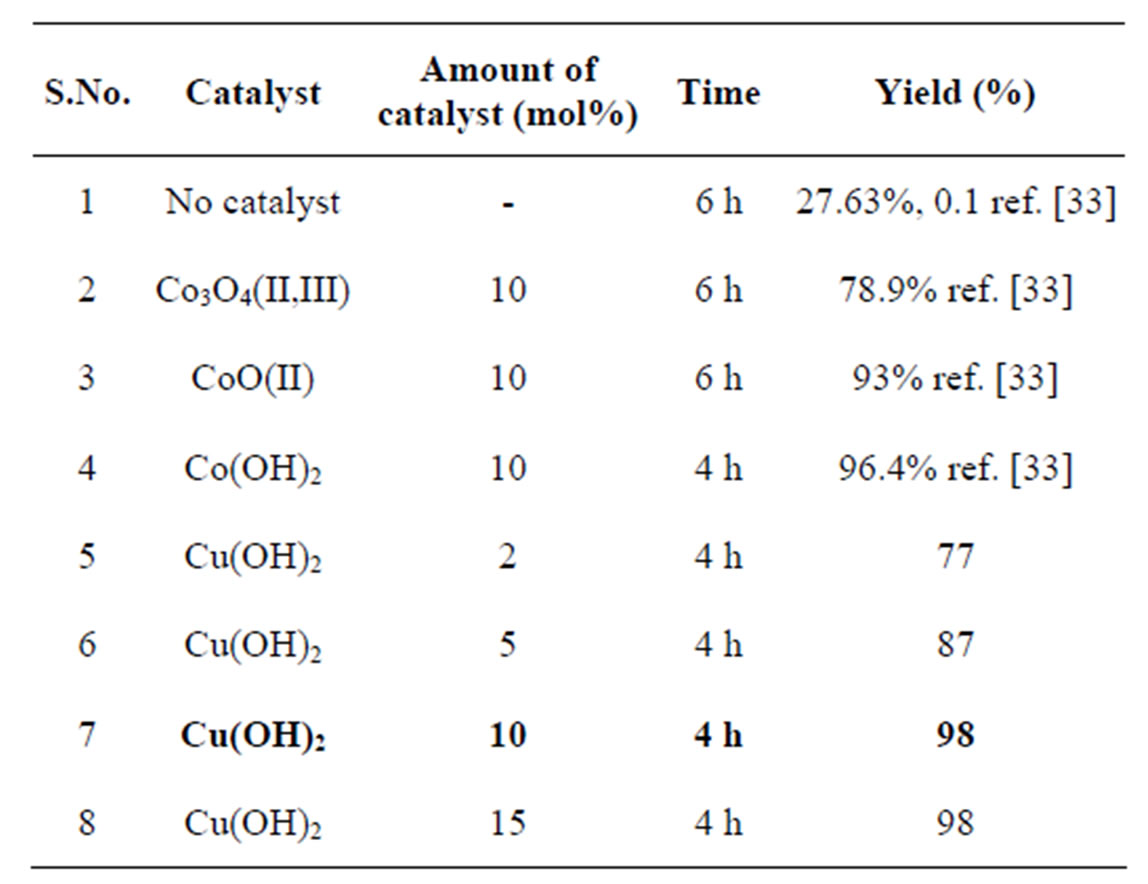
Table 1. Effect of the amount and catalytic activity of the various catalysts in the synthesis of multifunctionlised benzimidazoles.
Reaction Conditions: substrates: o-phenylenediamine (108 mg, 1.0 mmol), benzaldehyde (127 mg, 1.2 mmol), amount of catalyst = 10 mg, 10 mol%, reaction was conducted at room temperature in methanol as solvent.
observed that the amount of catalyst plays a significant role in controlling the activity of the catalyst. Among the various amounts of catalyst studied, 2 mol%, 5 mol%, 10 mol% and 15 mol% of Cu(OH)2 catalyst and 10 mol% of catalyst was found to be the best at room temperature in methanol condition and 2-phenyl-1H-benzimidazole 3 was isolated in high yield within short time (Scheme 1, Table 1).
In the case of Cu(OH)2 catalyst, the yields of the final product increases from 77% to 98% and whereas in the case of CoO(II) catalyst [33], the product yield increases from 72% to 93% with increasing the catalyst weight from 2 mol% to 10 mol%. The literature survey reveals that the 10 mol% Co3O4(II, III) activity [33] on this synthesis, gave yield 79% was lower than that by Co(OH)2 and CoO(II) solid catalysts. The order of activity was found as Cu(OH)2 > Co(OH)2 > CoO(II) > Co3O4. It was found that the present reaction readily proceeds, whereas no simple oxidation of aldehyde was observed.
The effect of the solvents affecting the catalytic activity of the copper hydroxide was also investigated under the optimized reaction conditions. Among various solvents like dichloro methane, methanol, acetonitrile and ethanol used for this transformation, methanol showed the highest yield (98%) in 4 h and was found to be the best solvent. Acetonitrile (94%) and ethanol (90%) exhibited almost the same activity and dichloro methane showed poor activity (40%). As per a plausible mechanism, the reaction proceeds via the activation of aldehyde 2 by Cu(OH)2 followed by imine formation A and this resulting imine further reacts with another -NH2 group of o-phenylenediamine resulting in the formation of dihydroimidazole B which subsequently undergoes aromati-
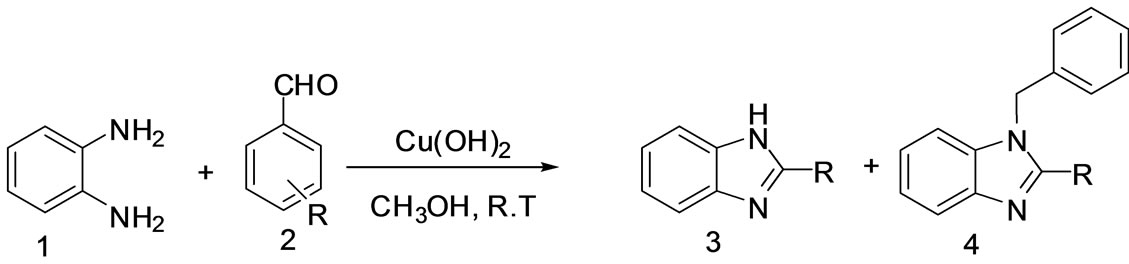
Scheme 1. Room temperature synthesis of multifunctionlised benzimidazoles using copperhydroxide solid catalyst.
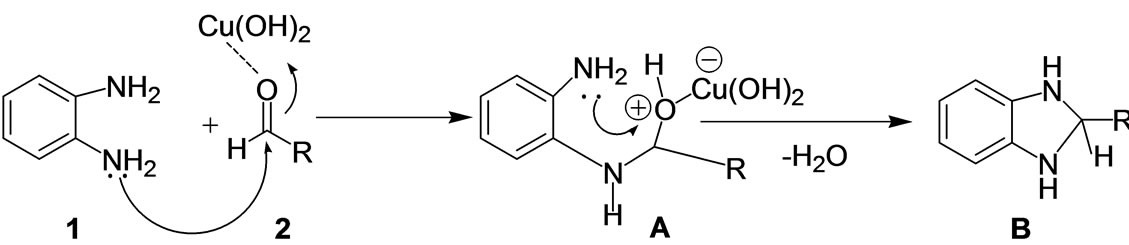
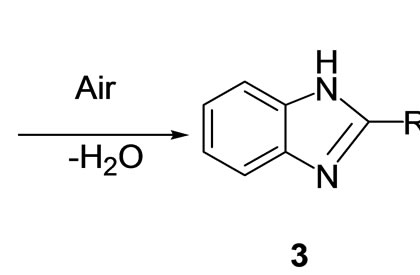
Scheme 2. A plausible mechanism for the synthesis of multifunctionalised benzimidazoles using Cu(OH)2.
zation under the oxidative conditions to give the benzimidazole 3 as shown in Scheme 2.
Encouraged by these results, we examined several substituted aldehydes including aromatic, heteroaromatic and aliphatic groups, which underwent smooth conversion to afford a wide range of benzimidazoles (Table 2). Aromatic aldehydes containing both electron-donating and electron-withdrawing groups worked well in this reaction. Unfortunately metal hydroxides are not explored much in the synthesis of various benzimidazoles. It was found that transition metal oxide is more effective for oxidation than the corresponding zero-valent metal powders. Open air and copper hydroxide both may be sources for the oxidation process. Here, it seems that copper hydroxide solid catalyst is acting as bifunctional like Lewis acid as well as oxidative reagent.
Many heterogeneous catalysts [32,33] and expensive Co(III) salen complexes [48] were used in the synthesis of various benzimidazoles. But all they have not achieved the products using aliphatic aldehydes. The present catalyst is simple, recyclable, green catalyst and more convenient than many catalysts presented in the above introduction. These catalysts were also found to be very active in the synthesis of benzimidazoles using an acid sensitive furfuraldehyde (3f) and bulky naphthaldehyde (3g), also worked well. Various substituted aldehydes have been used with similar success to provide the corresponding benzimidazoles in high yields (80% - 99%).
Finally, recycling experiments were conducted to find out the stability and reusability of the catalysts after the reaction. The catalyst was easily separated by centrifuge and reused after drying at 100˚C for 4 h. The efficiency of the recovered catalyst was verified with the Entry a, Table 3. Using the Copper hydroxide fresh catalyst, the yield of product 3a was 98%, while the recovered catalyst in the three subsequent cycles gave the yield of 94%, 90% and 88% respectively (Table 3).
In addition we have also carried out scale up experiments with 1.08 g of o-phenylenediamine and 1.27 g of benzaldehyde using 10 mol% of Copper hydroxide catalyst at room temperature in methanol. The Cu(OH)2 catalyst gave 98% of product 3a, that is almost similar to the yield obtained in Table 2 Entry a, indicating that the catalyst could be easily used on a large scale. In the present work, the catalytic activity of the Cu(OH)2 has been studied for synthesis of multifunctionalised benzimidazole derivatives at room temperature. It has been found that these commercially available catalysts are very cheap, recyclable and showed superior activity at room temperature than Co(III) salen [48], and cheaper than many other expensive heterogeneous catalysts [32,33]. All the synthesized compounds (3a-3v) colour, melting points were checked and characterized by using IR and 1H-NMR spectra data was given below.
3. Conclusion
In summary, benzimidazole and its derivatives were synthesized by the coupling of o-phenylenediamine with various aldehydes using commercially available Cu(OH)2 as an efficient recyclable solid catalyst under open oxygen atmosphere at room temperature using methanol as solvent. Various multifunctionalised Benzimidazoles could successfully be synthesized by using the Cu(OH)2 catalyst with acid sensitive, sterically hindered and substituted aromatic and aliphatic aldehydes. The reactions were performed in methanol and the catalyst could be reused for several cycles without much decrease in activity. The salient features of the present method include mild conditions, short reaction times, high yields, recyclable catalyst, large scale synthesis and simple procedure. These catalysts could replace the existing homogeneous and expensive heterogeneous catalysts which are currently being used in the synthesis of various industrially important and biologically active benzimidazoles.
4. Experimental Section
All chemicals and solvents were obtained from Aldrich and Spectrochem Pvt.Ltd., and used without further purification. Column chromatographic separations were carried out on silica gel of 60 - 120 mesh size. Melting points were determined on an open capillary apparatus and are uncorrected. The infrared absorption spectrums were obtained by using Perkin Elmer, Spectrum FTIR spectrophotometer with substances being pressed in a KBr pellet. The 1H NMR spectra of samples were recorded on a JEOL 400-MHz NMR spectrometer using TMS as an internal standard in DMSO-d6. The 1H chemical shift
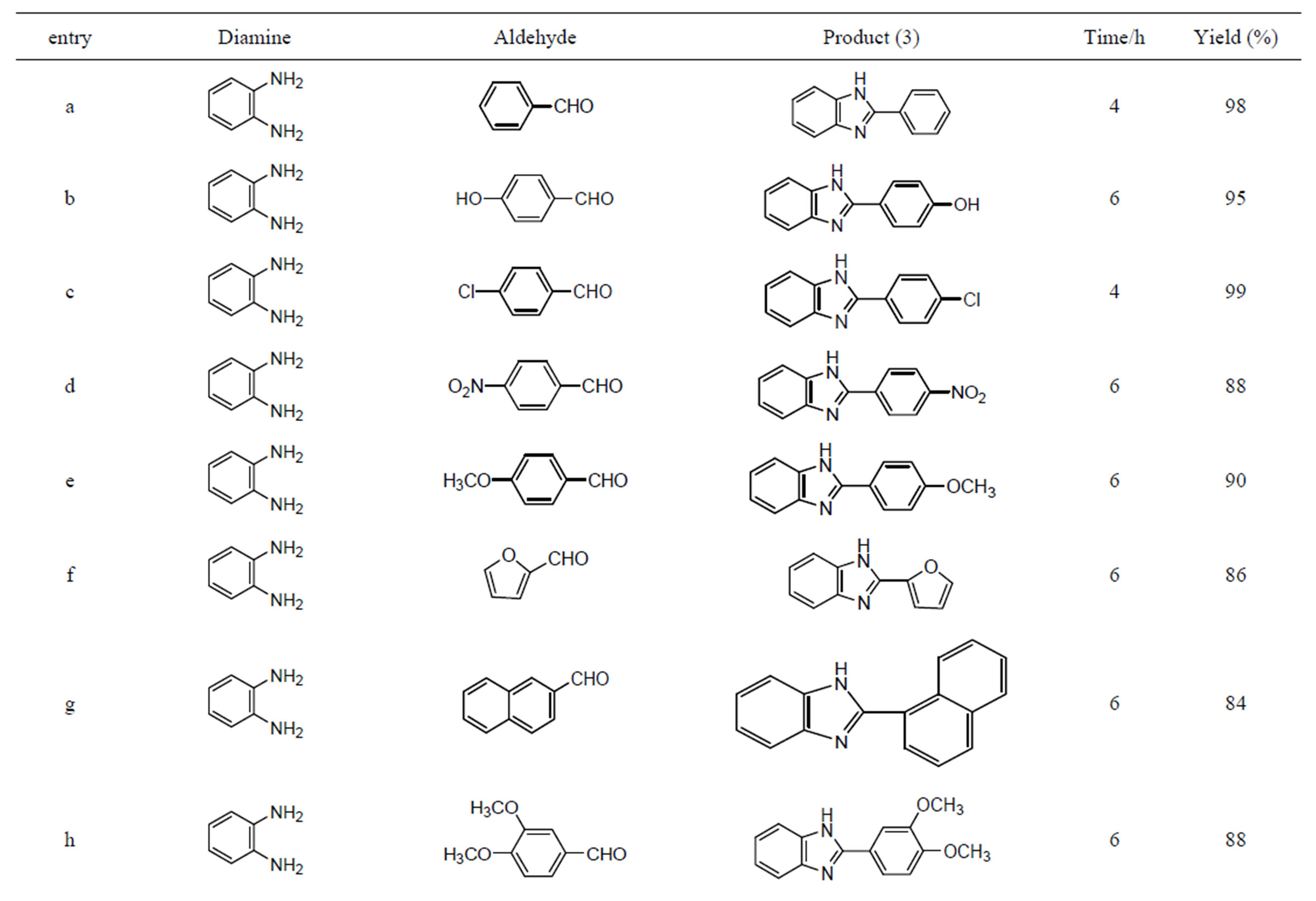
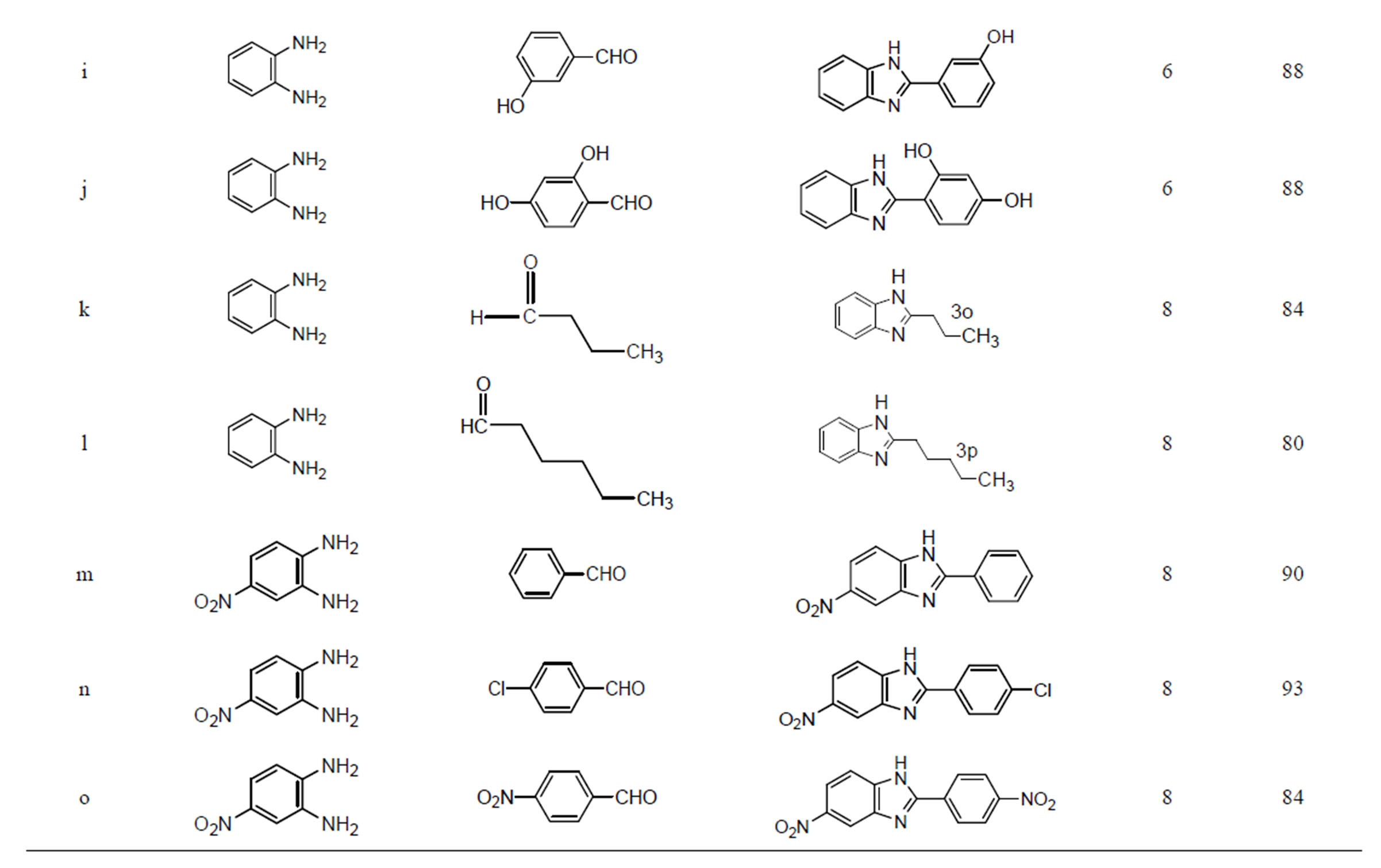
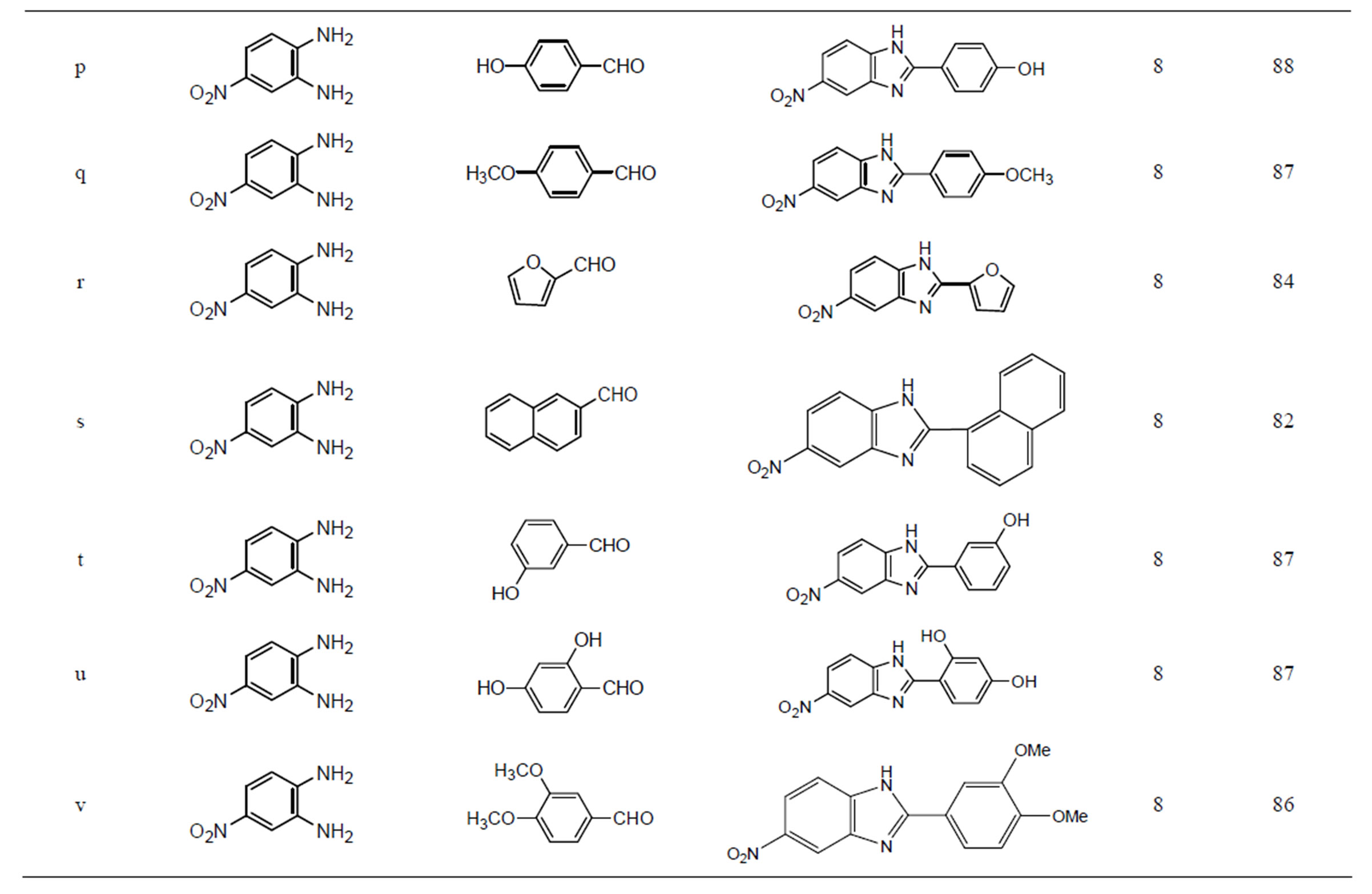
Table 2. Synthesis of multifunctionalised 2-substituted benzimidazoles using Cu(OH)2.
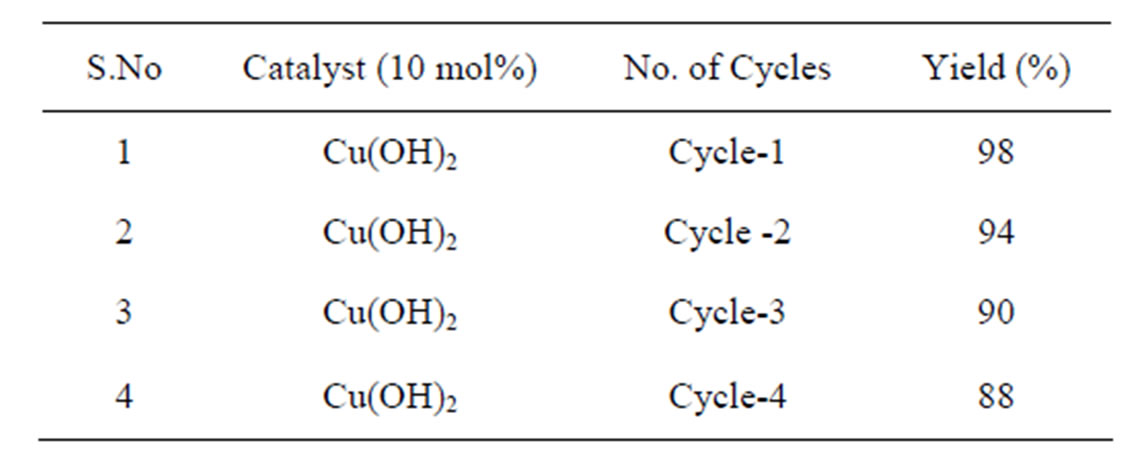
Table 3. Recycle performances in the synthesis of multifunctionalised benzimidazole 3a using Copper hydroxide as recyclable solid catalyst.
values were reported on the δ scale in ppm, relative to TMS and DMSO-d6 as solvent. Coupling constants (J) are reported in hertz (Hz).
General Procedure
To a mixture of o-phenylenediamine (1.0 mmol) and aldehyde (1.2 mmol) in methanol (3 mL) under open oxygen atmosphere, 10 mol% of the solid Copper hydroxide catalyst were added. The resulting mixture was stirred at room temperature for appropriate time (Table 2). After completion of the reaction, as monitored by TLC, the reaction mixture was diluted with methanol (20 mL) and the catalyst was separated by filtration. The organic layer was concentrated under reduced pressure and the crude product was purified by silica gel column chromatography using ethyl acetate-n-hexane (1:9) as eluent to afford pure benzimidazole product. Some of the compounds spectral data are given below. 3a: 2-Phenyl-1H-benzimidazole (Tables 2, 3a): Solid, yield: 98%, 0.190 g; m.p. 295˚C - 297˚C; IR (KBr): νmax 3039, 2964, 1460, 1410, 1309, 958, 708 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 7.17 - 7.23 (m, 2 H) 7.44 - 7.54 (m, 3 H) 7.56 - 7.63 (m, 2 H) 8.19 (dd, J = 8.17, 1.34 Hz, 2 H) ppm. 3l: 2-Pentyl-1H-benzimidazole (Table 2, 3l): Solid, yield: 80 %, 0.15 g; m.p. 158˚C - 160˚C; IR (KBr): νmax 3049, 2866, 2675, 1622, 1424, 1273, 1020, 750 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 0.85 (t, J = 6.95 Hz, 3 H, CH3) 1.28 - 1.44 (m, 4 H CH2, 2x) 1.81 - 1.94 (m, 2 H, CH2) 2.93 (t, J = 7.68 Hz, 2 H, CH2) 7.21 (dd, J = 5.85, 3.17 Hz, 2 H) 7.26 (s, 1 H) 7.55 (br. s., 1 H) 10.19 - 11.03 (m, 1 H, NH) ppm.
NOTES