1. INTRODUCTION
Gram-positive bacteria of the genus Bacillus are widely appreciated as industrial workhorses for the production of various enzymes owing to their ability of secreting proteins into the extracellular medium [1]. Among the huge number of commercial enzymes, proteases are of great importance for their extensive applications in detergent, tanning, food processing, silk degumming, medical diagnosis, bioconversion, waste treatment, and peptide synthesis [2-5]. Currently, proteases have accounted for up to 60% of the total enzyme sales in the global market, in which alkaline proteases represent the largest portion [6].
The wide applications of alkaline proteases stimulate a considerable research interests in them. Biosynthesis of these enzymes is controlled by various regulation systems in microorganisms, such as quorum-sensing and two-component systems [7,8]. In this case cells can regulate gene expression and activation of proteins in response to a large variety of environmental signals including nutrients and cell density [9]. Therefore, the production of enzymes is strongly influenced by medium components, including C/N ratio, easily metabolizable sugars, and nitrogen sources [10-14]. Besides, previous studies have shown that metabolizable carbon sources (such as glucose) and organic nitrogen sources, which are rich in amino acids and short peptides, display strong catabolite repression to protease production when used at higher concentrations [12,15,16]. Thus, development of a new strategy of evading catabolite repression to improve the alkaline protease production is of great necessity.
In the previous study, a strain Bacillus sp. B001 has been reported to produce a high level of extracellular protease about 34,277 U/mL and suggested its potential application in various industrial processes [17]. However, it is hard to further enhance the protease production by optimization of the available medium gradients of the original medium because the use of glucose, tryptone, and yeast extract causes catabolite repression on protease production [17]. To release the effect of catabolite repression and increase the protease production of Bacillus sp. B001, this study was proposed to develop a raw materials based (RMB) strategy using a statistical approach. Its potential applications for the protease purification and characterization and enzyme production by other Bacillus strains were further investigated.
2. MATERIAL AND METHODS
2.1. Chemicals and Raw Materials
All the chemicals used in present study were of the analytical grade. The azocasein was purchased from Sigma, USA. The hydrolyzed wheat gulten (HWG) was kindly donated by Wuhan Chortle Bio-chem Techonology Co., Ltd., China. Other cheap raw materials used in this study were obtained from Liangshan Ketai Biological Products Co., Ltd., Shandong, China, and Jining Fuqiang Materials Co., Ltd., Shandong, China.
2.2. Bacterial Strains and Culture Conditions
Bacillus sp. B001 was routinely grown on GYM medium comprising ingredients (g/L): glucose 10.0, tryptone 5.0, yeast extract 5.0, KH2PO4 1.0, MgSO4 0.2, Na2CO3 10.0, and agar 20.0 for solid medium [17]. B. subtilis W168, B. amyloliquefaciens TA208, were grown on LB medium (comprising ingredients (g/L): peptone 10.0, yeast extract 5.0, NaCl 10.0, and agar 20.0 for solid medium). B. clausii DSM 8716 obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) was grown on TSB medium (comprising ingredients (g/L): peptone from casein 17, peptone from soymeal 3, glucose 2.5, NaCl 5, KH2PO4 2.5, MnSO4 1, MgCl2 1, NaHCO3 4.2, Na2CO3 5.3, and agar 20.0 for solid medium) [18].
For seed culture, a loop of bacteria from the agar plate were transferred into a 250 mL Erlenmeyer flask containing 20 mL of the seed medium, and incubated at 30˚C and 200 rpm until the OD600 reached about 8.0. Unless specified otherwise, 1 mL of the seed culture was inoculated into 50 mL fermentation medium in a 500 mL Erlenmeyer flask. The compositions of fermentation medium were varied according to the experimental designs. Fermentation was performed at 34˚C and 200 rpm. All of the experiments were carried out in triplicate.
2.3. Screening Raw Carbon and Nitrogen Sources
The effects of different raw carbon and nitrogen sources on protease production of B001 were evaluated by substituting the carbon (glucose) and nitrogen (yeast extract and tryptone) sources in the original GYM medium with different raw carbon (soluble starch, corn flour, and wheat bran) and nitrogen (cottonseed cake flour, soybean meal, soy peptone, and HWG) sources at different concentrations.
2.4. Optimization of Medium Components by Experimental Design
Three stages of statistical experimental designs were used to optimize medium components for high level of protease production by Bacillus sp. B001. The levels of factors and experimental design matrixes were performed as described before except for some modifications [19,20]. Firstly, a 25-1 fractional factorial design (FFD) was employed to select the most important nutrients affecting protease production. Secondly, the method of steepest ascent was adopted to rapidly drive the variable concentrations from the initial estimate values to the proximity of the actual optimum values according to the regression coefficients obtained from the FFD. Finally, central composite design (CCD) of response surface methodology (RSM) was performed to investigate the optimal level of significant medium components affecting protease production.
2.5. Protein Purification and SDS-PAGE Analysis
The cell-free supernatant of fermentation cultures was collected and precipitated by (NH4)2SO4 to 80% saturation, and the mixture was incubated overnight at 4˚C. The precipitate was collected by centrifugation at 22,500 × g for 30 min, dissolved in TGN buffer (50 mM Tris-HCl, 50 mM NaCl, 5% glycerol, pH 8.0) and dialyzed against the same buffer to remove the residual ammonium sulfate at 4˚C. The crude extract was purified to be homogeneous through anion exchange DE52 chromatography (Whatman, England) with a linear gradient (0 - 1 M) of NaCl in the same buffer and the active fractions were pooled and stored at 4˚C for further analysis. Protein concentration was determined by the Coomassie Brilliant Blue dye method using bovine serumalbumin as a standard [21]. The cell-free supernatant, the crude mixture, and the purified protein were subjected to SDS-PAGE which was performed on a 5% stacking and a 12% resolving gel. The protein bands were visualized by staining with Coomassie brilliant blue R250.
2.6. Protease Activity Assay
Protease activity was measured using azocasein as substrate by a modified method of Deng et al. [17]. The absorbance at 440 nm was measured using the same reaction mixture with inactive enzyme as negative control. One unit activity was defined as the amount of enzyme that catalyzes the release of 1 μmol azo-dye per minute.
2.7. Effects of NaCl, Detergents, and Organic Solvents
To determine the effects of NaCl and detergents on enzyme activity, the assay was carried out at standard conditions (50 mM Tris-HCl, 5 mM CaCl2, and 1% azocasein, pH 8.5) containing 0 - 4 M NaCl or 1% (w/v) commercial available detergents. The endogenous proteases in the detergents were inactivated by boiling the diluted detergents for 30 min at 100˚C. The enzyme activity assayed in the absence of NaCl or detergent was taken as 100%. To determine the effects of NaCl, detergents, and organic solvents on enzyme stability, purified enzyme was incubated with 0 - 4 M NaCl, 1% detergents, or 50% solvents for 24 h at 20˚C before the residual activity was measured. The enzyme activity assayed under the same condition without incubation with NaCl, detergent, or solvent was set as 100%.
2.8. Statistical Analysis
The Design-Expert version 8.0.6 software package (StatEase, Inc., Minneapolis, MN, USA) was used for the experimental designs, regression analysis of the experimental data, and plotting response surface graphs. Statistical analysis of the model was performed to evaluate the analysis of variance (ANOVA). The quality of the regression model was estimated by the coefficient of determination R2, and its significance was determined by Fischer’s test. The statistical significance of the regression coefficients was tested by Student’s t-test. Finally, the optimum concentrations of the variables were calculated by differentiation of the quadratic equation [22]. All the experiments were carried out in triplicate.
3. RESULTS AND DISCUSSION
3.1. Effects of Various Culture Conditions on the Protease Production
The optimal pH and temperature for protease production were determined to be 11.0, and 34˚C, respectively. Among different carbon sources tested (Figure 1), maximum enzyme yield of approximately 23,689 was achieved in corn flour, followed by glucose (21,593 U/mL), soluble starch (18,570 U/mL), and wheat bran (2,185 U/mL). Among various nitrogen sources, soy peptone was selected as the best suitable material favored alkaline protease production (43,185 U/mL). Similar enzyme yields were observed with soybean meal (35,407 U/mL)

Figure 1. Effects of different carbon and nitrogen sources on the protease production by Bacillus sp. B001. Each carbon or nitrogen source was tested at different concentrations to get the maximum protease production.
and cotton cake flour (32,311 U/mL). While, lower enzyme yields were obtained when HWG (23,919 U/mL), yeast extract (21,593 U/mL), and tryptone (16,622 U/mL) were supplied as sole nitrogen source. Besides, MgSO4, KH2PO4, and CaCl2, which were added and optimized together in the flowing steps, could enhance the protease accumulation (data not shown). It has been shown that metabolizable carbon sources and organic nitrogen sources rich in amino acids and short peptides may repress enzyme production when higher concentrations were applied [12,15,23-25]. In order to relieve catabolite repression, Beg et al. [24] adopted an intermittent derepression and induction fed-batch strategy by separating biomass accumulation and protease production phases. In another case, citric acid was used to substitute glucose as the carbon source for alkaline protease production form B. licheniformis DSM 1969 [23]. In this study, we proposed to introduce a raw material based culture strategy on the basis that raw materials contained complex polymeric nutrients which were slowly hydrolyzed to metabolizable substrates by exoenzymes, thus high concentrations of which may be adopted without exerting catabolite repression. Therefore, corn flour and soy peptone were screened as suitable raw carbon and nitrogen sources enhancing proteases production of B001.
3.2. Optimization of Medium Components by Experimental Design
The most important medium components for the protease production were determined to be corn flour, soy peptone, and KH2PO4 by 25-1 fractional factorial design (Tables 1 and 2). A modified first-order model equation fitted to the data (Table 2) was regressed to the equation below (Eq.1):

Table 1. Applied levels of independent variables in the 25-1 FFD.

Table 2. 25-1 FFD design matrix with corresponding results.
 (1)
(1)
where  is the predicted enzyme production; x1, x2, x3, and x4 represent the coded values of corn flour, soy peptone, MgSO4, and KH2PO4, respectively. Then, the method of steepest ascent experiment was further carried out to move near to the optima of the three significant components, the maximum production of 61,800 U/mL was obtained at run 4 as shown in Table 3 (16.20 g/L corn flour, 5.85 g/L soy peptone, and 1.13 g/L KH2PO4). Consequently, this medium was chosen for further RSM optimization.
is the predicted enzyme production; x1, x2, x3, and x4 represent the coded values of corn flour, soy peptone, MgSO4, and KH2PO4, respectively. Then, the method of steepest ascent experiment was further carried out to move near to the optima of the three significant components, the maximum production of 61,800 U/mL was obtained at run 4 as shown in Table 3 (16.20 g/L corn flour, 5.85 g/L soy peptone, and 1.13 g/L KH2PO4). Consequently, this medium was chosen for further RSM optimization.

Table 3. Experimental design and results of the steepest ascent path.
According to the steepest ascent path results, a central composite design was performed to determine the optimal levels of the three important medium components. The coded and real values of variables at various levels are listed in Table 4. The experimental design and corresponding enzyme production of the central composite design were presented in Table 5. By applying multiple regression analysis on the experimental data (Table 5), a polynomial model was obtained as Eq.2:
 (2)
(2)
where Y is the predicted enzyme production; x1, x2, and x4 is the coded values of corn flour, soy peptone, and KH2PO4, respectively. The model F-value of 59.69 with P-value less than 0.0001 indicated that the model was highly significant according to the analysis of variance. The results of ANONA for the full polynomial quadratic model were listed in Table 6. All terms in the model were statistically significant because their P-values were less than 0.05, which demonstrated that corn flour, soy peptone, and KH2PO4, as well as their quadratic terms had significant effects on alkaline protease production. The fitness of the model was examined by the coefficient of determination R2, which was calculated to be 0.9817, revealing a good correlation between the experimental and predicted values, i.e. above 98% of variability in the response could be explained by the established model. Therefore, the model is supposed to be adequate to predict the maximum levels of the variables and the corresponding response.
The 3D response surface were plotted to get a better description of the interactions between variables. As shown in Figure 2, each contour curve represented an infinitive number of combinations of two test variables with the other one held at the center point. The smallest ellipse in the contour diagram represented the maximum predicted values [26]. The interactions between corn flour and soy peptone, corn flour and KH2PO4, and soy peptone and KH2PO4 were depicted in Figure 2. All the

Table 4. Coded and real values of variables in central composition design.

Table 5. Experimental design and results of central composite design.
contour plots showed in Figure 2 were elliptical, demonstrating perfect interactions between the three independent variables [27], which was in well agreement with their low P-values obtained in previous statistical analysis (Table 6).
The optimum concentrations of the variables were calculated by solving the regression equation Eq.2. The optimal values of each test component in coded units were as follows: x1 = 1.403, x2 = 0.661, x4 = −1.121. Thus, the actual concentration of corn flour, soy peptone and KH2PO4 were 19.60, 6.20, and 1.16 g/L, respectively.
3.3. Enhanced Protease Production of Strain B001 in Optimized Medium
Three validation experiments were performed to verify the accuracy of the model by using statistical optimized medium: corn flour 19.60 g/L, soy peptone 6.20 g/L, KH2PO4 1.16 g/L, MgSO4 0.12 g/L, CaCl2 0.60 g/L, and Na2CO3 10.0 g/L. An enzyme production of 63,200 U/mL was obtained at the fermentation time of 27 h (Figure 3(a)), which was in agreement with the predicted maximum value of 62,539 U/mL calculated according to Eq.2. As shown in Figure 3(a), the increase of protease production correlated with the biomass accumulation for 24 h. The cells then entered into the stationary phase and the maximum enzyme production of 63,200 U/mL was reached at 27 h. After that time, the enzyme yields were relatively stable despite the decrease in biomass.
The protease production showed three improvements compared with the previous condition [17]: 1) the protease production of Bacillus sp. B001 was 1.84 folds higher than the previous value, 2) the fermentation time for maximum protease production was three hours earlier than the previous conditions, and 3) a relatively stable enzyme production was observed in the stationary and declining phases in the optimized medium which is of great significance in industrial fermentation, because this improvement makes the enzyme harvest time wider. These improvements could be attributed to the slow release of available nutrients into the medium though hydrolyzing corn flour and soy peptone by the enzymes secreted by Bacillus sp. B001, preventing the repressive effects of high concentration of catabolites. In addition, the utilization of corn flour and soy peptone as economical raw materials in fermentation decreases the production costs. Taken together, these results have shown that the optimized fermentation medium could sustain a stable, high yield of protease produced by Bacillus sp. B001 over a longer period of fermentation. To the best of our knowledge, the protease yield achieved in the optimized medium is much higher than the results obtained by previous shake-flask fermentation [10,17, 28,29]. Besides, the cost of medium was cut down by using inexpensive raw materials which is of great importance, because the cost of medium is one of the major obstacles against the successful application of any tech-

Table 6. Analysis of variance of the full second-order quadratic model.
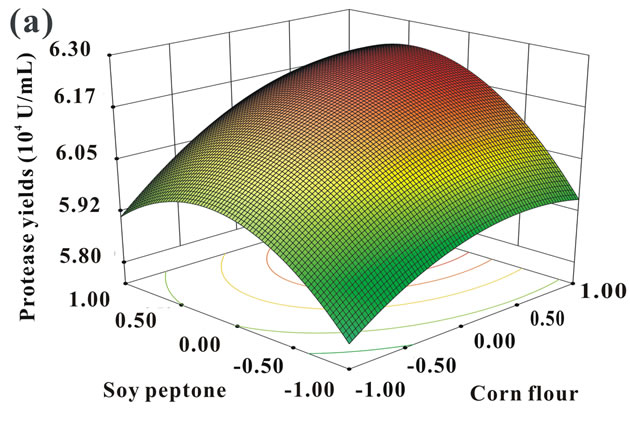


Figure 2. 3D response surfaces and corresponding contour plots indicating the mutual effects between soy peptone and corn flour (a), KH2PO4 and corn flour (b), and KH2PO4 and soy peptone on protease production (c).
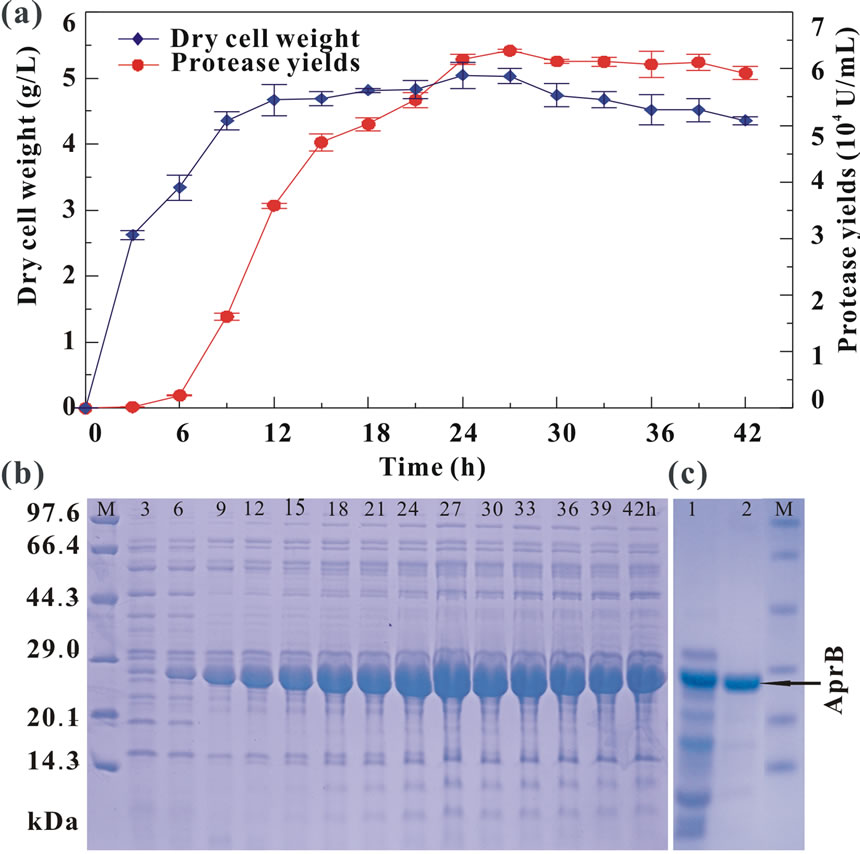
Figure 3. Time course of the protease production (a) and SDSPAGE analysis of the extracellular (b) and purified (c) proteases from B001 under optimized condition. Lane M, protein marker; Lanes 3 to 42, the total proteins from cell-free supernatants for each 3 h interval. Lane 1, crude proteins precipitated by (NH4)2SO4; Lane 2, protein purified by anion exchange.
nology in the enzyme industry [2,11].
3.4. Purification of Extracellular Protease
The protease in the fermentation supernatant was also characterized by SDS-PAGE (Figure 3(b)). The majority of extracelluar proteins were near to a molecular weight of approximately 27 kDa, the amount of which varied in accordance with protease activity at different stages of growth (Figure 3(a)). The protease yield and purification fold of 23.6% and 3.8, respectively, were obtained by ammonium sulphate fractionation followed by anion exchanger (Table 7). Protein purification was successfully achieved to the homogeneity as evident by a single band corresponding to 27 kDa on SDS-PAGE (Figure 3(c)). Compared with the previous study in which the extracelluar protease yield of B001 was 5.6% [17], the purification procedure in this study not only achieves a higher yield, but also an easier process. Therefore, extracellular protease produced in the optimized culture accounted for the majority of extracellular proteins and would make the following purification process much easier.
3.5. Effects of Salt and Detergents on Protease Activity and Stability
The purified protease remained active and stable with most of the compounds tested (Figures 4(a) and (b)). In the presence of 1 M NaCl, the enzyme retained 72.5% of relative activity. A rapid decrease was observed with 2 and 4 M NaCl. However, the enzyme retained above 100% residual activity after incubation with 1 - 4 M NaCl for 24 h. The effects of NaCl on the protease activity were similar with that on rSAPB activity; however, its salt-stability was higher than rSAPB and similar with the triple mutated SAPB-L31I/T33S/N99Y [30]. Therefore, the extracellular protease from B001 displays high salt-stability making enzyme keep stable during the granulation process of detergents formation.
As shown in Figure 4(b), purified protease from B001 showed relative high activity and stability in the presence of commercial laundry detergents. It exhibited more than 100% activities in the presence of Keon and Ariel, followed by Cnice (75.3%), Tide (69.4%), and OMO (37.8%). After incubated with various detergents for 1 h, the enzyme also retained more than 100% activity in Tide and Ariel, and displayed 96.4%, 89.8%, and 66.9% activities when exposed to Cnice, Keon, and OMO, respectively. The highly stability towards salt and good compatibility with commercial laundry detergents of protease from B001 gives strong support for its application as detergent additives.
3.6. Effects of Organic Solvents on Protease Stability
To determine the effects of solvents on protease stability, Butanol (logP = 0.88), Hexamethylene (logP = 3.2), N-hexane (logP = 3.5), DMSO (logP = −1.35), Acetonitrile (logP = −0.15), Methanol (logP = −0.76), Ethanol (logP = −0.24), Isopropanol (logP = 0.05), Xylene (logP = 3.1), Benzene (logP = 2.0), and Acetone (logP = 0.2) at concentration of 50% (v/v) were selected. As shown in Figure 4(c), the enzyme retained more than 100% activity in various tested solvents with logP < 4.0. It is well known that the solvent with logP < 4.0 is considered to be extremely toxic because of their higher degree of partitioning into the aqueous layer which disrupts the hydrogen bonding and hydrophobic interactions and results in loss of enzyme activity [31]. However, solventstable enzymes usually contain a greater number of negatively charged acidic amino acids on the surface which keep the protein soluble either by preventing the protein aggregation via electrostatic repulsion or by forming hydrated ion networks with cations [32]. Therefore, the excellent tolerance to solvent of the protease from Bacillus sp. B001 might be attributed to its high ratio of negative charged amino acids which accounts for 21.24% surface [33]. This remarkable property substantiates promising application of enzyme as a biocatalyst for peptide synthesis in low water systems.

Table 7. Purification of extracellular protease from Bacillus sp. B001.
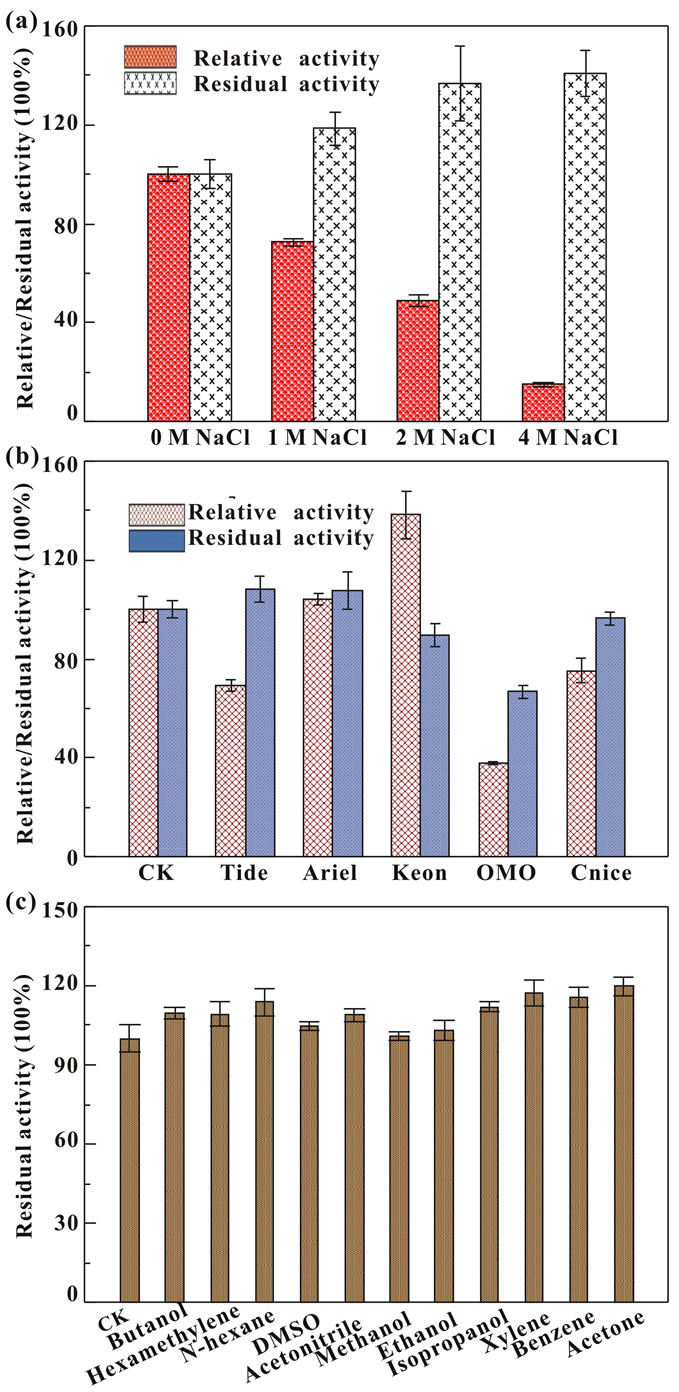
Figure 4. Effects of salt (a), commercial detergents (b), and organic solvents (c) on protease.
3.7. Effects of Medium on the Extracelluar Protease Production of Other Bacillus Strains
The raw material based culture strategy was also applied for producing protease by other Bacillus strains using the optimized medium. As shown in Figure 5, the culture strategy significantly enhanced extracelluar protease production of B. subtilis, B. amyloliquefaciens, and B. clausii by 2.9, 8.5, and 3.2 times, respectively. The effective application of the culture strategy in Bacillus strains could be attributed to the effect of the crude materials corn flour and soy peptone on the release of the catabolite

Figure 5. The extracellular proteases production of Bacillus strains with raw material based culture strategy.
repression common in all bacteria stains. Therefore, the raw material based culture strategy, which displayed promising advances in effectively enhancing the exoenzyme yield and cutting down the medium cost, is an effectively method that might be wildly applied in the enzyme production in various Bacillus strains, even in other genus.
4. CONCLUSION
In this study, a raw material based culture strategy was developed to increase the protease production by Bacillus strains. Cheap corn flour and soy peptone were screened as the optimum raw materials and optimized by statistical experiment designs. Under the optimized conditions, the protease productions of Bacillus sp. B001 and other Bacillus strains were significantly increased 1.8 - 8.4 folds. Additionally, protease from the optimized fermentation cultures of B001 was quickly purified to homogeneity by ammonium sulphate precipitation and anion exchange. The purified enzyme exhibits superior tolerant to high salt, commercial laundry detergents, and a wide range of organic solvents. Taken together, a lowcost, easy-purified, and effective producing strategy was developed in this study, which represented a potential application for enzyme production by various bacteria strains.
5. ACKNOWLEDGEMENTS
Financial supports from the National Hi-Tech Research and Development of China (2007AA02Z212) and the Key Project of Chinese Academy of Sciences (KSCX2-YW-G-075-22) were gratefully acknowledged.
NOTES