Regulative effect for natural killer cell by hot spring hydrotherapy—Quantitative and qualitative discussion ()
1. INTRODUCTION
Despite our defense system, the overwhelming problems of possessing our dual system, the innate and adoptive do not seem to guard or even prevent the development of one internal threat to survival. Moreover, every individual exposes to the risk of immunodeficiency in daily life with both internal and externals. Especially, NK cell is first line of defense against virus infected cell and/or tumor cell. So a cancer patient requires to select appropriate menu to regulate immune function as in quantitative as well as qualitative even for low pathogenic microorganisms.
The factors that influence the acquired immune activity are systemic metabolic disorder such as diabetes, malnutrition, extreme exhaustion, stress, aging and medical side effect in cancer [1-10]. So we have to select appropriate menu to regulate immune function through leukocyte storage. The menu had been summarized and listed as CAM: complementary and alternative medicine [11-18].
Many systems are in place to evaluate Western therapies that aim at healing the symptoms of an illness. However, when the purpose of a therapy is to enhance the QOL of healthy people, such as some alternative medical therapies, no widely-accepted evaluation system has been established. To fill this lack, we would like to propose the number and functions of leukocyte subsets as indicators for the evaluation of alternative therapies.
In this report, we would like to focus on the effect of hot spring hydrotherapy, especially in regulatory effect of natural killer cell in number and function.
2. MATERIALS AND METHODS
2.1. Subjects and Methods
Twelve to 25 individuals per group were tested from the spring in 2005 to autumn. For hormonal analysis, all the volunteers were informed and consented by the ethics committee of Kanazawa Medical University. According to the percentage of lymphocytes or granulocytes in the total leukocytes, the hydrotherapy group was further divided into two types: subjects whose granulocyte number was over 60% belonged to the G type, those whose lymphocyte number was over 40% belonged to the L type (Table 1).
The hot springs selected for these tests were Wakura Onsen Spa (a conc. sodium chloride with chlorinated soils, Nanao City, Ishikawa Pref.), Chugu Onsen Spa (a dil. sodium chloride with sodium carbohydrate, Hakusan City, Ishikawa Pref.) with a Japanese style of bathing that free from under wear. The water temperature was 41 degrees centigrade, fluctuating up or down by 1 degree centigrade.
On the first day of the trial, five-milliliters of blood were drawn from the forearm vein of all the subjects at 4 o’clock p. m. During that evening and the next morning, the hydrotherapy group bathed in the hot spring two to three times, for 20 minutes each time. Finally, at 4 o’clock p. m. on the second day, the blood of all the subjects was sampled again, avoiding a circadian rhythm of leukocyte subset, granulocyte and lymphocyte [19-21]. Heparin was used as an anti-coagulating reagent (Figure 1).
2.2. Leukocyte Count
We accessed each variation of leukocyte with the data avoid circadian rhythm of leukocytes. The total number of leukocytes was recorded with a standard counter. In the differential counting, 200 cells were counted in each

Figure 1. Experimental protocol. The age of the volunteers ranged from 18 to 87 (n = 25). They were separated into two groups, 35 years old and younger, and 36 and older, according to the results in this text. One group took a bath two to three times for 20 min, within a 24 hr period. Blood was collected from a peripheral venula before hydrotherapy and 24-hrs later. The blood was prepared as plasma and examined by a FACScan. Details of the procedures are within the text [23].
May-Grunwald-Giemsa stained smear. The numbers of granulocytes, coenocytes and lymphocytes were determined, respectively.
2.3. Lymphocytes and Lymphocyte Subset Analysis
The whole blood obtained from the subjects was washed twice with PBS (phosphate buffered saline, pH 7.2). The suspensions were treated with fluorescent monoclonal antibodies (FITC-conjugated anti-human CD2, CD4, CD8, CD16, CD19 and CD56) separately. After 30 minutes of staining at 4 degrees centigrade, the cells were analyzed by a FACScan (Becton Dickinson Co. Ltd. U.S.A.).
2.4. Cytokine Containing Cell Analysis
The blood cell suspensions were cultured with PMA (phorbol 12-myristate 13-acetate), Ionomycin and BSA (bovine serum albumin) for 4 - 5 hours at 37 degrees centigrade. After that, the cell suspensions were stained using the monoclonal antibodies of PE-IL-4, FITC-IFN-g and FITC-IL-1β, respectively. Then they were analyzed by the FACScan (Becton Dickinson Co. Ltd. U.S.A.). The antibodies and reagents used in the entire test were purchased from Becton Dickinson Immunocytometry system (U.S.A.).
2.5. Hormonal Level Analysis
The serum obtained from the subjects was used for hormonal analysis. The concentration of adrenocorticotropic hormone (ACTH), triiodothyronine (T3), thyroxine [13] and thyroid stimulating hormone (TSH) were measured by radioimmunoassay (RIA). Adrenalin, noradrenalin and dopamine were measured by high performance liquid chromatography (HPLC). The examination of these hormonal level was ordered in Ishikawa Health Service Association (Institution by Public Organization, Ishikawa, Japan).
2.6. Statistical Analysis
The statistical comparisons between two groups (before and after hot spring hydrotherapy) for the test of significant difference were performed using paired t-test and wilcoxon signed-ranks test. Further, the test of the correlation were performed a spearman’s correlation coefficient by rank test. Data are expressed as means ± standard error of mean (SE). A P value <0.05 was considered to be statistically significant.
3. RESULTS
3.1. The Effect of Hot Spring Hydrotherapy on the Leukocyte Count Correlated with the Age and Original Basic Count of the Individuals
We accessed each variation of leukocyte with the data avoid circadian rhythm of leukocytes [19-21]. Table 1 shows the total numbers of leukocytes, granulocytes and lymphocytes from peripheral blood collected before and after hot spring hydrotherapy in the old ager and young ager [22]. The quantitative variation of the two groups differed. In the young ager, the total number of WBC (p < 0.05) and granulocytes (p < 0.01) clearly decreased. On the other hand, the total number of WBC and lymphocytes significantly increased (P < 0.01) after hydrotherapy in the old ager [22]. Furthermore, the results show that changes in the leukocyte count and subset count had a negative relationship before and after hydrotherapy. In other words, subjects who had a higher cell count level before hydrotherapy tended to show a decrease in the number of WBC 24 hrs after hydrotherapy. There was a significant correlation between age and rate of change (p < 0.01). Since the turning point got at 35 years old, we separated the participants into a young ager (under 35) and an old ager (over 36) group (Figure 2).
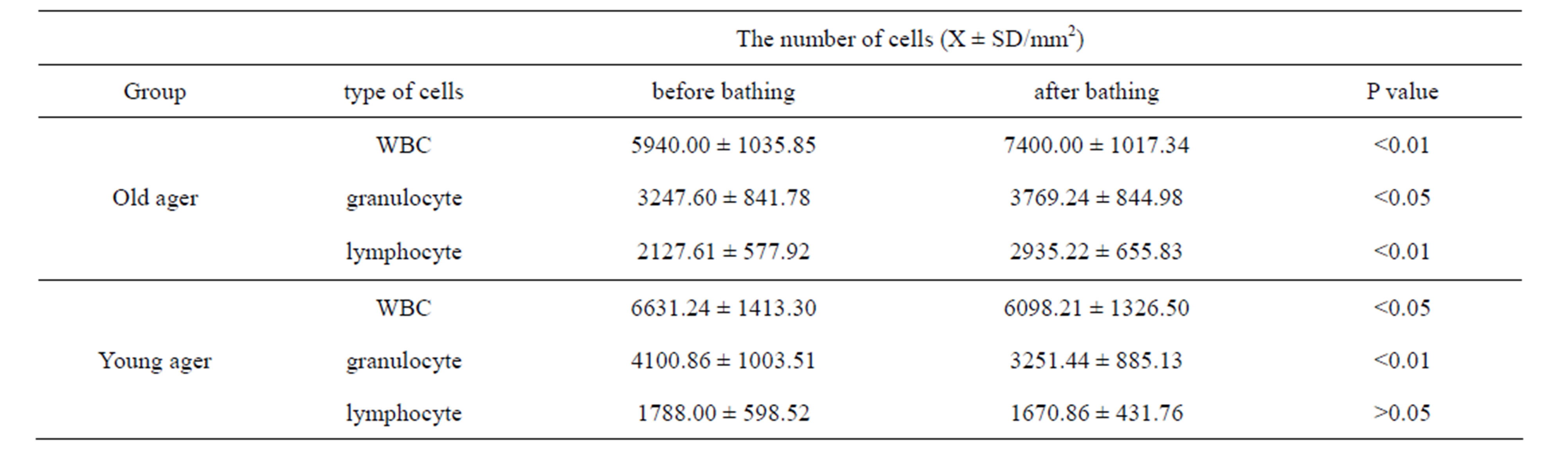
Table 1. The regulation by HSH in number of WBC according to age. The volunteers were divided into two groups, 35 years old and younger, and 36 and older, corresponding to the up-regulation or down-regulation of peripheral WBC in the text [23]. The WBC subsets were then counted morphologically according to granulocyte and lymphocyte number.
3.2. The Effect of Hot Spring Hydrotherapy on the Number of Lymphocyte Subsets Also Correlated with the Original Basic Number of the Individuals
The results varied among the lymphocyte subsets before and after hot spring hydrotherapy. After hydrotherapy, the CD16+, CD8+ and CD19+ cells increased in the young ager. Meanwhile, the CD16+ cells increased in the old ager while the CD19+ cells decreased remarkably (P < 0.01). This quantitative change in lymphocytes are shown in Figure 3 Except for CD8+ cells, the CD2+, CD4+, CD16+, CD19+ and CD56+ cells, all showed regulative variations. There was a strong correlation between the variable ratio and the value before hydrotherapy, high values tended to decrease and low values tended to increase in CD2, CD4 and CD16 (r = 0.692, r = 0.558, r = 0.488, p < 0.001), next are CD19, CD56 (r = −0.260, r = −0.225). Only CD8 cell did not alter the number in the day before (r = −0.150).
3.3. Hot Spring Hydrotherapy Affected the Functional Changes in Cytokine-Containing Cells
To test whether hot spring hydrotherapy affected the functional maturation of immunocytes within a short time, we further investigated the number of cytokine containing cells by FACS analysis. This method reveals cytokine producing cell number by peering off the surface of lymphocyte, enable to express cells in festival evening, compare than serum cytokine level that correspond to paper tips of post festival.
Figure 4 shows the effect of hot spring hydrotherapy on cytokine production. Even though the increase in IFN-g containing cells had statistical significant(r = −0.323, P < 0.01), the increase in IL-4 was remarkable (r