Screening Test for Antibiotics in Medicinal Plants (STAMP): Using Powdered Plant Materials Instead of Extracts ()
1. Introduction
Infections by multi-drug resistant (MDR) microorganisms are increasing [1], and the number of fully active antibiotic options currently available to treat these infections is extremely limited [2]. Moreover, infections associated with biofilm-forming microorganisms increase exponentially [3,4], representing challenges even for new classes of antifungal compounds such as the echinocandins, lipid formulations of amphotericin B (AMB), and the new triazoles. According to Coates et al., [5] the major problem nowadays is that no matter how potent a new antimicrobial compound is, its therapeutic effectiveness will be relatively short due to the inevitable resistance developed by microorganisms.
It is evident that the pipeline for the development of new, effective antibiotics with activity against MDR organisms looks grim [6]. Soon we may have no medications available in our therapeutic arsenal to treat these highly resistant microbes. This current state of urgency motivated some authors to investigate an innovative approach for inhibition of MDR microorganisms: herbal extracts [2]. In fact, many medicinal plants are used for treatment of infections worldwide [7,8]. A number of in vitro studies have shown antimicrobial activity of herbal extracts, and several clinical trials have been performed to date [7].
There are approximately 350,000 plant species in the world [9]. With the current methods, it will take very long before we can screen all of them for all desired pharmacological properties. While all these are being discussed, many areas are being degraded right now and many species will be extinct before being screening for pharmacological properties. Thus, the development of new, accessible, cheap, fast, and simple methods for screening plants for pharmacological effects is highly desirable. It would allow every country in the world, even the poor ones, to start looking into their own vegetation for new medicines. This would have potential to substantially change medical care, as we know it today.
We hypothesized that it is possible to screen plant species for antimicrobial activity by using powdered plant materials instead of plant extracts. We named this method as screening test for antibiotics in medicinal plants (STAMP).
2. Material and Methods
2.1. Reagents and Solvents
NaCl, ethanol and dichloromethane (all analytical grade) were purchased from Synth (Diadema, SP, Brazil), methanol (HPLC grade) was from JT Baker (USA), ampicillin, amphotericin B, fluconazole, dimethyl sulfoxide (HPLC grade), CDCl3 (99.96% D), RPMI-1640 media were purchased from (Sigma-Aldrich, São Paulo, SP, Brazil), gentamicin sulfate was from Ourofino® (Ribeirão Preto, SP, Brazil), bacteriological agar, Sabouraud dextrose, Mueller-Hinton were purchased from Himedia (Curitiba, PR, Brazil). The microorganisms (all ATCC) were purchased from Fundação André Tosello (Campinas-SP, Brazil). Spectra: Spectrophotometer Unico (USA). HPLC system: from Shimadzu (Kyoto, Japan), namely an LC-10-AVP instrument equipped with an auto-injector SIL-10AF, a photodiode array detector SPD-M20A, and a Phenomenex (Torrance, CA, USA) Luna® C18 column (250 × 4.6 mm i.d.; particle size of 5 µm).
2.2. Plant Materials
In the experiment we used several different species, as follows: Ocimum gratissimum L. (Lamiaceae), Ocimum basilicum L. (Lamiaceae), Ocimum selloi Benth. (Lamiaceae), Cordia curassavica (Jacq.) Roem. & Schult. (Boraginaceae), Alternanthera brasiliana (L.) Kuntze. (Amaranthaceae), Physalis angulata L. (Solanaceae), Tinospora cordifolia (Thunb.) Miers (Menispermaceae), Baccharis dracunculifolia D.C. (Asteraceae), Baccharis trimera (Less.) D.C. (Asteraceae), Lippia sidoides Cham. (Verbenaceae), Rapanea leuconeura (Mart.) Mez. (Primulaceae, five different genotypes: 5R1, 5R2, 5R3, 5R4, and SA10), Cochlospermum regium (Schrank.) Pilg. (Bixaceae), Eugenia punicifolia (Kunth) D.C. (Myrtaceae) and Peritassa campestris (Cambess.) A.C. Sm (Celastraceae). All plants were grown without any pesticides or chemical fertilizers (organically) in gardens.
These species were chosen to be representative of different families, genera, and geographic regions. In addition, we used different plant parts from all studied species—namely, roots, aerial parts, and inner bark. Parts of one of the investigated species, R. leuconeura, were collected from individuals of five different genotypes (Table 1). All specieswerecollected inJardinópolis City (São Paulo State, Brazil—Latitude: 21˚01′04″, Longitude: 47˚45′50″, altitude: 590 m)in February 2011. The P. campestris was collected in Ribeirão Preto City (São Paulo State, Brazil—Latitude: 21˚10′40″, Longitude: 47˚48′36″, altitude: 544 m).
Exsiccates of these plants were deposited at the Herbarium of the University of Ribeirão Preto (UNAERP) vouchers, O. gratissimum (HPMU 1329), O. basilicum (HPMU 1455), O. selloi (HPMU 1456), C. curassavica (HPMU 1457), P. angulata (HPMU 1458), T. cordifolia (HPMU 1459), B. dracunculifolia (HPMU 1460), B. trimera (HPMU 1426), L. sidoides (HPMU 1461), R.

Table 1. List of plant species, plant organs, and mass of powdered plant materials used in the wells.
leuconeura (HPMU 1462), C. regium (HPMU 1463), E. punicifolia (HPMU 1464) and P. campestris (HPMU 1414).
To confirm the method’s efficiency we evaluated dry powdered plant materials, and compared with wet and hydro-alcoholic extracts of each species, and also the triterpenoids maytenin (tingenone) and netzahualcoyone (Figure 1) isolated from P. campestris.
2.3. Preparation of Powdered Plant Materials
The raw plant material was dried in oven at 45˚C under circulating air, ground in a knife mill and sieved to standardize the particle size (48 mesh). The mass of dry powdered plant material deposited in each well was calculated from its density and the volume of the well (Table 1).
2.4. Preparation of the Wet Powdered Plant Materials
The dry powdered plant material was added to distilled water with pipette plastic tips (1:5 w/v, Figure 2). The tips were then wrapped in aluminum sheets and autoclaved for 15 min at 1 atm and 121˚C. The proportion of powder and water yielded the resulting mixture to be pasty rather than liquid. The resulting mixture was then called wet powdered plant material. It was not called aqueous extract because it was not filtered. This was done 24 h before the assays to avoid evaporation of the water. All the mass obtained in this step was used in the wells.
2.5. Preparation of the Hydro-Alcoholic Extracts
The hydro-alcoholic extracts (ethanol:water, 1:5 v/v) were obtained by sonication (3 times of 20 min). After each run, the solution was filtered and more solvent was added to the tart (tart:solvent, 1:2 w/v). After maceration and filtration the extracts were evaporated and lyophilized. The lyophilized extracts were added to the plastic tips to allow the insertion into the wells. The amount of lyophilized hydro-alcoholic extract used was that required to fill the well completely.
2.6. Isolation of Maytenin and Netzahualcoyone and Analysis of Hydro-Alcoholic Root Extract of P. campestris
Maytenin and netzahualcoyone were isolated from root barks of P. campestris as described elsewhere [10]. The identities of the two isolated compounds were confirmed by comparison of their spectroscopic data (UV, MS, 1H and 13C and 2D NMR) with literature values [11,12].
The hydro-alcoholic root extract of P. campestris and the chemical standards, maytenin and netzahualcoyone, were dissolved in methanol (2.5 mg/ml and 1.0 mg/ml, respectively), filtered through a 0.22 µm membrane of a nylon filter, and analyzed by High Performance Liquid Chromatography-Diode Array Detector (HPLC-DAD). The mobile phase was 85:15 (v/v) metanol:water (with 0.1% formic acid) under isocratic mode at a flow rate of 1 ml/min. The injection volume of the sample and standards was 20 µl and the total analytical run time was 30 min. The spectral data were collected over 30 min in the 200 - 800 nm range, and the chromatograms were analyzed and plotted at 420 nm. Compounds present in the sample were identified by comparing retention time (Rt) of the standards.
2.7. Screening for Antimicrobial Activity
The STAMP method was developed in agar diffusion (6-mm wells) on 14-cm Petri plates containing 60 ml of Mueller Hinton growth medium (pH 7.6). The microorganisms used were Candida albicans ATCC 10231, Candida parapsilosis ATCC 22019, and Staphylococcus aureus ATCC 6538. Inoculum adjustment was done in 0.85% saline solution and absorbance was measured with

(A) (B) (C)
Figure 1. Antifungal activity of different preparations of Cochlospermum regium against Candida albicans in Mueller Hinton growth medium with pH 7.6. Legend: (A) dry powdered plant material; (B) Wet powdered plant material; (C) Hydroalcoholic extract.

Figure 2. STAMP steps: (A) Preparation of plastic tip to hold the powdered plant materials and lyophilized hydro-alcoholic extracts. (B) Weighting the sample within the tip. (C) Wrapping the tip with aluminum sheet. (D) Inoculation of the microorganism using a sterile swab. (E) and (F) Opening 6-mm wells using upside-down plastic tips. (G) and (H) Introduction of the sample in the well.
a spectrophotometer at 530 nm with transmittance of 72% - 75% (yests) and at 530 nm with absorbance of 0.10 - 0.15 (bacteria). All tests were based on the approved standards CLSI M2-A9 (2006) and M44-A (2003), for bacteria and yeasts, respectively.
The powdered plant materials that exhibited antibacterial activity were also assayed for antibacterial activity in Mueller Hinton medium with a pH of 9.0.
Microorganisms were inoculated into the plates by using a sterile swab that was inserted into the solution containing the microorganism, already adjusted, and twisted several times. Next, the swab was pressed against the wall of the tube to remove solution in excess. The microorganisms were then plated on three different positions so that all the plate’s extension was fully covered.
After inoculation of the microorganisms, the 6-mm wells were opened (Figures 2(E) and (F)). Next the powdered plant materials (wet and dry, autoclaved and non-autoclaved), and the lyophilized hydro-alcoholic extracts were added to the wells, as shown in Figure 2.
The plates were then kept in oven with circulating air at 37˚C for 24 (bacteria) or 48 (yeasts) h before reading. Readings were done by measuring the halo of inhibition, including the wells, in millimeters.
The standard antibiotics (positive controls) used were fluconazole and ampicillin, both mixed with autoclaved starch (excipient) in concentration of 1 mg/g. A total of 28 mg of the mixture was added to the positive control wells, according to its calculated density. All the experiments were performed in triplicate.
2.8. Minimum Inhibitory Concentration
Minimum inhibitory concentrations (MIC) were determined according to standards CLSI M7-A6 (2003) for bacteria and CLSI M27-A2 (2002) for yeasts. The highest concentration used was 100 µg/ml. The microorganisms used were Candida albicans ATCC 10231, Candida parapsilosis ATCC 22019 and Staphylococcus aureus ATCC 6538. All the experiments were performed in triplicate. Positive controls were amphotericin B and gentamicin sulfate.
2.9. Statistical Analysis
All results were expressed as means and standard deviations (SD). No hypothesis test was done.
3. Results
Several dry powdered plant materials were effective against S. aureus, namely P. angulata, R. leuconeura (5R2, 5R3, and 5R4), C. regium, P. campestris, and E. punicifolia. These effects were also seen for the correspondent hydro-alcoholic extracts, except for P. angulata (Table 2). None of the wet powdered plant materials was effective against S. aureus, except for P. campestris.
For C. albicans, many different dry and wet powdered plant materials showed antifungal activity: O. selloi, L. sidoides, R. leuconeura (5R1, 5R2, 5R3, 5R4, and SA10), C. regium, P. campestris, and E. punicifolia. The antifungal activity was also observed for the correspondent

Table 2. Antimicrobial effect of the different species and preparations against Staphylococcus aureus. Results are expressed as the mean diameter of the halo (mm).
hydro-alcoholic extracts (Table 3).
Similarly, for C. parapsilosis, many different wet and dry powdered plant materials also showed antifungal activity: L. sidoides, R. leuconeura (5R1, 5R2, 5R3, 5R4, and SA10), C. regium, P. campestris, and E. punicifolia. The antifungal activity was also observed for the correspondent hydro-alcoholic extracts (Table 4).
When compared to the results obtained with the hydroalcoholic extracts, the STAMP method was able to identify 10 of the 11 species with activity against C. albicans, plus one, yielding a sensitivity of 91%, and a specificity of 86%. For C. parapsilosis, screening with powdered plant materials correctly identified all nine species with potential antifungal activity that were also identified by the hydro-alcoholic extract. Surprisingly, the use of powdered plant materials identified three other species with potential antifungal activity, yielding sensitivity of 100% and specificity of 67%. For the activity against S. aureus, however, the method was able to identify only six of the 12 species identified with hydro-alcoholic extracts, plus one.
After these results, we sought to investigate whether the low performance of the STAMP method for antibacterial activity could be explained by the low solubility of the compounds in a pH of 7.6. We then repeated the experiments for S. aureus, by this time using a Mueller Hinton growth medium with a pH of 9.0. The results are summarized in Table 5. The alkaline pH increased the activity of E. punicifolia, and the antibacterial effect of R. leuconeura (SA10) was identified, which was not evident in a pH of 7.6. As a result, for S. aureus the method was able to identify seven of the 12 species identified with hydro-alcoholic extracts, plus one, rendering a sensitivity of 64%, and a specificity of 86%. In general, the higher the medium’s pH, the greater the observed antimicrobial activity (Figure 1). The species with antimicrobial activity identified for each microorganism are summarized in Table 6 and Figure 3.
HPLC-DAD chromatogram of the hydro-alcoholic root extract of P. campestris gave two major peaks

Table 3. Antimicrobial effect of the different species and preparations against Candida albicans. Results are expressed as the mean diameter of the halo (mm).

Table 4. Antimicrobial effect of the different species and preparations against Candida parapsilosis. Results are expressed as the mean diameter of the halo (mm).

Table 5. Activity of the dry powdered plant material against Staphylococcus aureus in Mueller Hinton growth medium with a pH of 9.0. Results are expressed as the mean diameter of the halo (mm).
(Figure 4) at retention times (Rt) of 8.7 min and 10.1

Figure 3. Proportion of plant species with antimicrobial activity among all tested species, according to different microorganisms and extracts.

Figure 4. Chromatogram of Peritassa campestris hydro-alcoholic root extract by HPLC-DAD analysis. Peak identification: maytenin (1) and netzahualcoyone (2). Conditions: mobile phase: 85:15 (v/v) metanol:water (with 0.1% formic acid); injection volume: 20 μL; detection λ = 420 nm; flow rate: 01.0 ml/min.
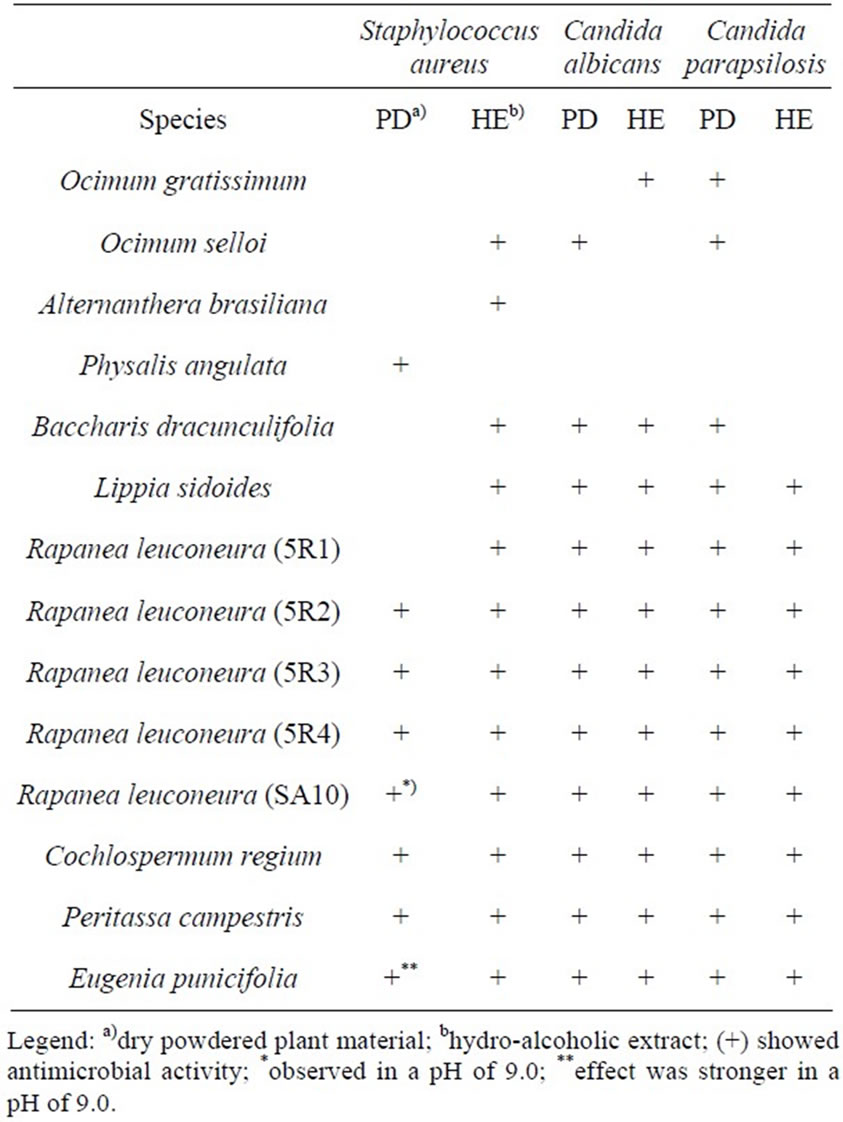
Table 6. Species identified as having antimicrobial activity according to the type of extract used.
which were identified as maytenin and netzahualcoyone, respectively, by matching the Rt with those of the corresponding standards. These substances showed antimicrobial activity mainly against S. aureus (MIC of 0.78 µg/ml), but also C. albicans and C. parapsilosis (Table 7).
4. Discussion
This is the first report on the use of powdered plant materials on screening tests for antimicrobial activity. We called this the STAMP method. We have shown that this method is best suitable for screening of plants with antifungal properties, since it has higher sensitivity and specificity compared to the use of hydro-alcoholic extracts. In addition, the method can also be used for screening of plants with antibacterial activity, as long as the pH is increased to 9.0.
In a similar experiment, Miyasaki et al. [2] screened 60 different plants for antimicrobial activity, and found that 18 of them had the desired effect. The lack of correlation between the amount of herbal extract required to fill the indented agar well and its ability to diffuse out of the well onto the Mueller Hinton agar plate were probably due to their different solubility in the aqueous environment and to the molecular size of its chemical constituents.
We have experienced a similar issue when testing for antibacterial activity. However, when we used a medium with an alkaline pH, the antibacterial activity of many species became evident. Interestingly, the alkaline pH totally reduced the activity of ampicillin against against S. aureus by half. The intracellular pH of microorganisms does not vary significantly in response to variations in extracellular pH. However, extracellular pH can affect the transport of certain compounds across the cell mem-

Table 7. Minimum inhibitory concentration (MIC, µg/ml) values of quinone-methide triterpenoids isolated from Peritassa campestris against Staphylococus aureus, Candida albicans, and Candida parapsilosis.
brane [13]. Therefore, it is evident that any alteration in pH can enhance the passage across the cell membrane of water-soluble secondary metabolites with antimicrobial activity, like flavonoids, anthocyanins, and alkaloids. According to other authors, any increase in pH can potentiate interactions between polyphenols and membrane surfaces through deprotonation of hydroxyl groups [14].
The antimicrobial activity of the isolated compounds from P. campestris confirmed the results obtained with STAMP method. P. campestris was chosen for the MIC experiment because it contains substances named quinonemethide triterpenoids, such as maytenin and netzahualcoyone, that are chemotaxonomic markers of the Celastraceae family, and that exhibit a myriad of biological activities [15,16]. The results we obtained with the powdered plant material of P. campestris using the STAMP method and the results of maytenin and netzahualcoyone using the MIC method were similar and showed greatest antimicrobial activity against S. aureus, followed by C. parapsilosis and C. albicans. These results were also similar to those obtained by Elhag et al. [17], with a similar low MIC against S. aureus (0.6 µg/ml). Regarding to the yeasts, a study by Gullo et al. [18] showed results similar to ours, with a lower MIC of maytenin for C. parapsilosis and higher for C. albicans (15.62 e 62.50 µg/ml, respectively). All these results support the work of Moujir et al. [19] showing that triterpenoids exhibit antimicrobial activity for Gram-positive bacteria and yeasts.
Our results are very promising because the technology required for the successful discovery, development, and production of botanical drugs is not yet in place, and efforts required for their emergence should be substantial [20]. The current method of drug discovery, the so-called high throughput screening (HTS), is not easily adaptable to complex mixtures produced from natural sources. This is mainly due to the high cost per sample, complexity of resupply, difficulty in isolation and characterization of actives, lack of reproducibility, and interference from compounds in complex mixtures [21]. In addition, isolation and purification of the active principles from an exceptionally complex matrix are one of the major bottlenecks affecting natural product discovery, and this relatively simplistic and reductionist approach may lead to inconclusive findings in clinical trials [20,22].
More recently the pharmaceutical industry has avoided three situations concerning the use of medicinal plant compounds in the formulation of bioproducts: a) isolation of pure substances from complex mixtures, which is expensive and requires long-term working; b) production of crude extracts, which present poor solubility; and c) use of species with reduced supply [23].
Many natural molecules, when isolated, are not watersoluble, and consequently have poor bioavailability. But if one takes the whole package, that is, the phytocomplex, the interaction between all the compounds present within the plant can actually increase solubility in water and bioavailability. In our experiment, the observed antimicrobial effect can only be attributable to water-soluble compounds, since we did not use any extraction technique and the media was aqueous. Therefore, by using this new screening method, we can be sure that the major compounds responsible for the pharmacological property will potentially have a fairly good bioavailability. Increasing the medium’s pH allowed the identification of additional species with the properties of interest.
Given the urgency of discovering new antibiotics, it is highly desirable to screen as many plant species as possible, but this can take a very long time. Many factors may increase the time needed for the screening of a plant. First, different plant parts can have different chemical compositions. Second, plants can have different chemotypes, resulting in different chemical profiles. Third, climate and soil type can alter the plant’s chemical composition. Lastly, different plant extracts with different solvents have to be obtained and tested. The preparation of different herbal extracts consumes time and both human and financial resources. We believe that the new method we present here can effectively contribute to the field, because it requires less time since no extraction method is needed. This will allow more cost-effective research, increasing the possibility of finding new drugs with antimicrobial activity.
5. Conclusion
In conclusion, the STAMP method, which uses powdered plant materials instead of plant extracts, is a cheap, widely available, technically easy, time sparing, reproducible, and sensitive method of screening plant species for antimicrobial properties, and can significantly shorten the time and money spent during drug development. The method we propose selected many species with potential antimicrobial activity for future phytochemical investigations aiming at the relationship between chemical structure and biological activity. We have also shown that this method is suitable for screening different genotypes of a single species, and different plant organs.
6. Acknowledgements
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant# 2010/15168-6) for financial support and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We also thank to Msc. Camila Hernandes for her support on MIC experiments, and to Dalma M. S. Rodrigues, MD, for her invaluable contribution on manuscript revision.
NOTES