Substrate Effect on Plasmon Resonance of a Gold Nanoparticle Embedded Amorphous BaTiO3 Film ()
1. Introduction
Recently, nanoparticles (NPs) attracted interest of research community because of their drastic change in various physical properties like conductivity, magnetism, optical properties etc. It has got many applications in various fields like optoelectronics, plasmonics, nanophotonics, and optically chemical sensors and in biology. The change in properties is the consequence of high surface to volume ratio, defects on the surface and geometry of the NPs [1]. The concept of nanoparticles interactions and its interaction with substrate, consequences of various shape emergences in synthesis are still an interesting problem to be solved theoretically. The SPR (Surface Plasmon Resonance) concept is widely used for different kind of sensors like explosive detection and bio sensor [2, 3]. Gold NPs have been proved to be very useful in sensor applications [4]. The periodic patterns of Au NPs are very useful in THz generation which has application in explosive detection [4]. Also metal NP embedded dielectrics are of current interest in studying metal induced crystallization. The study of capped nanoparticles with different chemicals is of interest in fundamental as well as in application point of view. This is not yet understood completely. One of such problems is the thiol capped gold NP. This gave a new physical property to gold NP which is unexpected for gold. The study of charge transfer effect in thiol capped gold NP gives the understanding of unexpected magnetism in gold [5]. Thus depending upon the bonding between the gold NP surface atoms, the capped chemical gives a variety of properties to gold NP. The charge transfer from gold NP to the dielectric matrix is very important in explaining unusual properties like magnetism and enhanced nonlinearity in gold NP embedded dielectric films. Apart from this the gold NP embedded dielectric films (Au embedded TiO2) are very useful in understanding the laser induced damage mechanism [6]. Gold NP embedded BaTiO3 shows 10 times more enhanced third order optical nonlinearity (χ(3)) compared to gold dispersed SiO2 films at 532 nm [7]. BaTiO3 also exhibits multifunctional properties in the nanoscale. Therefore studying the gold NP dispersed BaTiO3 is still interesting. The localised surface plasmon resonance is very sensitive to the host effects. Here we have studied the substrate effect on the localised surface plasmon resonance of gold NPs dispersed BaTiO3 film. The size and shape dependence of surface plasmon resonance of nanoparticles motivated the development of different chemical methods for the synthesis of metal nanoparticles. The control over the shape and size and inter particle distance of nanoparticles is very difficult in chemical methods. There are some colloidal methods which can give control over the size but not on interparticle separation.
2. Experimental
We have used sol-gel method to tune the size of gold nanoparticles embedded in BaTiO3 films. Barium acetate, Titanium isopropoxide and Chloroauric acid were the precursor chemicals for the method. Barium acetate was dissolved in 40 ml of Propanoic acid and is stirred for 30 min. 2 ml of Titanium isopropoxide was dissolved in 10 ml of Ethyl alcohol and added drop by drop to the dissolved Barium acetate in the presence of N2 atmosphere. This was again stirred for 3 hrs [7]. Two kinds of samples were prepared by using different amount of Chloroauric acid. Sample (1) was prepared by mixing 0.025 gm of Chloroauric acid containing propan-2-ol to 50 ml of above prepared BaTiO3 sol. In the case of sample (2), the amount of Chloroauric was increased to 0.086 gm. This gold containing sol was used as coating solution. The films were deposited on borosilicate glass and fused silica substrates by spin coating (WS-400B-6NPP/LITE/8K) at 1000 rpm for 5 sec and 3000 rpm for 5 sec. To get sufficient thickness we have deposited six layers of films in each sample case. The films were dried at 200˚C for 2 hr, and then heated to 400˚C for 20 min. The micro structural characterization is carried out by using, Tecnai 20 G2 STwin, FEI electron microscope, operated at 200 KV. The optical properties were carried out using JASCO V-570 UV/VIS/NIR spectrophotometer.
3. Results and Discussion
Here we have studied the substrate and particle size effect on localized surface plasmon resonance. It is an important technique in the detection of various microscopic organisms and absorbed chemicals including trace explosives. The organisms or chemicals which are to be detected when come in contact with gold NP, the dielectric constant of host changes. The change in dielectric constant of host will give the shift in surface plasmon resonance. Thus the shift in peak gives the preliminary information about the organisms and chemical. Normally the shift in wavelength corresponding to absorption peak is highly dependent on particle size, shape and interparticle spacing, when the nanoparticles are put inside the matrix and the film is supported by substrate. In this case the plasmon absorption peak is also sensitive to the dielectric constant of matrix and the substrate on which the NP containing film is deposited [8]. Mohammad et al. explained substrate and surrounding matrix effect on surface plasmon resonance by finite difference time domain simulation (FDTD) [9]. Here in this case the size and periodicity of particles is not well defined, hence the FDTD analysis will not help in analyzing the data of plasmon resonance.
Figures 1(a) and (b) show the TEM images of sample (1) on glass and fused silica substrate respectively. It is also clear that the gold NP’s are of average 5 nm size. Similarly 1(c) and 1(d) shows for sample (2) that the average size of gold nanoparticles is 10 nm on glass and fused silica substrates respectively. The optical absorption spectrum in Figure 2 shows localised plasmon resonance peaks for sample (1) (2(a) on glass, 2(b) on fused silica) and for sample (2) (2(c) on glass, and 2(d) on fused silica). In the case of sample (1), the particles are of 5 nm in size and the plasmon resonance peak appears at 414 nm. As the substrate changes to fused silica it shows a red shift in the absorption spectrum to 420 nm. While in the case of sample (2), due to the increase in the Chloroauric acid, the particles are of 10 nm in size and show absorption at 566 nm, and as the substrate changes to fused silica, it shows a small red shift to 568 nm.
 (1)
(1)
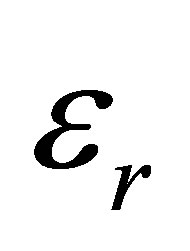 = real part of dielectric constant.
= real part of dielectric constant.  = imaginary part of dielectric constant.
= imaginary part of dielectric constant.  = dielectric constant of host medium. d = diameter of nanoparticle.
= dielectric constant of host medium. d = diameter of nanoparticle.

Figure 1. TEM image of gold NPs embedded in amorphous BaTiO3. Sample (1) (a) on glass; (b) on fused silica. Sample (2); (c) on glass; and (d) on fused silica.
Thus, as discussed above the increase in Plasmon absorption peak wavelength with particle size increment is in agreement with Mie theory. The Equation (1) given above will give the relation between particle size, dielectric constant of host medium, dielectric constant of nanoparticle and scattering cross section. Table 1 summarizes the shift in Plasmon peak with particle size and with substrate. Hence, it is clear that localized plasmon resonance is sensitive to the substrate change. Both the particle size and substrate effect playing an important role in deciding the Plasmon peak of gold NP’s in these films. The broad peak around 1000 nm may occur because of very small elongated gold NPs ~2 - 3 nm in diameter [10,11].
4. Conclusion
The understanding of gold-dielectric composite is of fundamental and technological importance. We successfully synthesized gold NP embedded in amorphous BaTiO3 film and studied the effect of substrate on localized surface plasmon resonance. The 5 nm Au NPs show a plasmon peak around 414 nm and particles with 10 nm show a peak around 566 nm. As per Mie’s theory it also shows red shift in Plasmon peak with increment in NP size. The films on the fused silica substrates show a red shift in the plasmon resonance peak compared to the films on the borosilicate glass substrates.

Figure 2. Absorption spectrum of gold NP embedded amorphous BaTiO3. Sample (1) (a) on glass; (b) on fused silica. Sample (2) (c) on glass; and (d) on fusedsilica.

Table 1. Plasmon absorption peak for 5 and 10 nm particles. Substrate and size effect on its plasmon resonance.
5. Acknowledgements
We would like to thank DRDO, for their financial support, and Centre for Nano Technology at UOH for providing TEM based material characterizing facility.
NOTES