Role of Lactic Acid Bacteria-Myeloperoxidase Synergy in Establishing and Maintaining the Normal Flora in Man ()
1. Introduction
In 1892 Döderlein suggested that fermentative lactic acid producing vaginal bacteria protected the host from pathogenic microbes [1]. The role of Döderlein’s bacillus, i.e., Lactobacillus acidiphilus, in healthy vaginal flora, was extended to include the normal intestinal bacterial flora of infants by Moro in 1900 [2]. At about the same time Tissier described the normal flora of breast-fed infants, establishing the importance of Bifidobacteria (aka, Tissier’s bacillus) [3]. In 1908 the health benefits of the lactic acid bacteria that constitute healthy human flora were popularized by Metchnikoff’s book, Prolongation of Life [4].
The lactic acid bacteria (LAB) are Gram-positive, nonspore forming microbes incapable of cytochrome synthesis and lacking the heme electron transport mechanisms required for efficient respiration. As such, LAB metabolism is fermentative. The LAB are acid-tolerant, aerotolerant and lack heme catalase activity [5]. The absence of cytochromes restricts LAB redox metabolism to flavoenzymes. The resultant fermentative metabolism produces acids, and in many cases, hydrogen peroxide (H2O2) [6]. LAB fermentative metabolism is essential to food preservation. In their role as the dominant microbes of the normal flora, LAB serve the innate host defense against pathogenic microbes.
Many LAB produce H2O2 in the presence of oxygen [7,8]. The viridans streptococci that comprise the normal mouth flora [9] produce sufficient H2O2 to cause the characteristic green, i.e., viridans, hemolysis seen on blood agar [10]. Streptococcus sanguinis and Streptococcus mitis are reported to produce H2O2 in the range of about 50 nanomoles (nmol)/min/mg of dry weight [11]. The rates of H2O2 production by Streptococcus oralis and S. sanguinis are described to be several nanograms/min /106 CFU [12].
2. Phagocyte Leukocyte Microbicidal Action
Neutrophil leukocytes and monocytes provide the innate phagocyte defense against pathogenic infection and are the microbicidal effector cells of the acute inflammatory response. Large quantities of neutrophils and monocytes are produced by the myelopoietic bone marrow and released into the circulating blood daily. Cytokines and chemotactic molecules direct phagocyte migration from the circulating blood to sites of infection where contact with opsonified microbes results in phagocytosis and formation of a phagosome. Fusion of the phagosome with lysosomal azurophilic granules containing cationic enzymes produces the phagolysosome. Both neutrophils and monocytes contain relatively large quantities of a cationic haloperoxidase, i.e., myeloperoxidase (MPO) [13], capable of oxidizing chloride (Cl−) to hypochlorite (OCl−) in an acid milieu containing H2O2 [14].
As depicted in the schematic of Figure 1, phagocytosis is linked to activation of NADPH oxidase. The activated oxidase catalyzes the NADPH-dependent reduction of molecular oxygen (O2) liberating the NADP+ required for increased hexose monophosphate shunt (aka, pentose pathway) dehydrogenase activity. This increased glucose metabolism is proportional to increased non-mitochond-
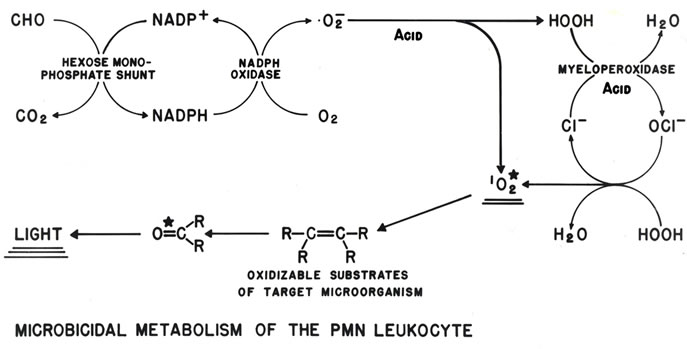
Figure 1. Schematic depiction of neutrophil production of the H2O2 that drives MPO oxidation of Cl− to OCl−, followed by reaction of the OCl− with an additional H2O2 to produce . The resulting microbicidal dioxygenation reactions yield excited carbonyl products that relax by photon emission, i.e., chemiluminescence [15].
. The resulting microbicidal dioxygenation reactions yield excited carbonyl products that relax by photon emission, i.e., chemiluminescence [15].
rial oxygen consumption. These metabolic activities are necessary for phagolysosomal acidification and H2O2 production, and provide an optimal milieu for H2O2- dependent MPO oxidization of Cl− to OCl− [15-18]. The reaction of OCl− with a second H2O2 yields electronically excited singlet molecular oxygen , a highly reactive but metastable electrophilic oxygenating agent with a microsecond lifetime. Both OCl− and
, a highly reactive but metastable electrophilic oxygenating agent with a microsecond lifetime. Both OCl− and  * are potent microbicidal agents.
* are potent microbicidal agents.
2.1. Phagocyte Production and Myeloperoxidase
Each day the bone marrow of a healthy human adult releases about a hundred billion neutrophils and monocytes into the circulating blood [19]. Approximately 5% of the dry weight of each neutrophil is MPO [20], a 145 kDa dimeric alpha-heme haloperoxidase carried in the neutrophil's azurophilic granules [13]. The average cell volume of a neutrophil is about 450 femtoliter (fL); its specific gravity is 1.1, and its cell water content is about 84% (16% dry weight). As such, each neutrophil contains about 4 femtograms (fg) MPO. Thus, the total MPO released per day is about 0.4 mg. Nanomolar concentrations (0.1 - 1.0 µg/mL) of MPO are highly microbicidal when presented with physiologically available chloride and sub-millimolar concentrations of H2O2 [21].
2.2. Tissue Neutrophils and MPO Availability
The circulating lifetime of neutrophils in the blood is less that twelve hours, and is followed by a less well characterized tissue phase that can last for a few days [19]. Although neutrophilia, i.e., influx of neutrophils, is a characteristic feature of acute inflammation, small numbers of neutrophils are known to routinely migrate into the alimentary tract spaces, bladder and body cavities in the absence of inflammation. Continuous migration of neutrophils into the mouth [22] and vagina [23] has been well documented. Relatively large numbers of neutronphils (105) can be lavaged from the mouths of healthy humans. The quantity of neutrophils present in fluid lavaged from the mouth is proportional to the subject's circulating blood neutrophil counts [22]. Inflammation and G-CSF treatment increase neutrophil production as well as the quantity of MPO per neutrophil [24,25].
Migrating tissue phase neutrophils transport sufficient MPO to body flora spaces to have an effect on the microbial flora. The presence of MPO might be expected to exert microbicidal action against the resident H2O2-producing LAB [26,27], but the opposite is observed.
3. MPO Selectivity Binds and Kills Microbes
Direct MPO binding to bacteria can be visually demonstrated by contacting bacteria in suspension with MPO, and then pelleting the bacteria by centrifugation. The degree of bacterial pellet staining is proportional to the MPO bound [21]. MPO was found to bind to all of the Gram-negative, and most of the Gram-positive bacteria tested, with the exception of viridans streptococcus, i.e., S. sanguinis. The degree of MPO binding was quantified by reduced-minus-oxidized difference spectral analysis and by chemiluminescence-based Scatchard analysis. Both methods confirmed and expanded the visual observation of selective MPO binding. With the exception of the fermentative LAB, all bacteria tested showed high degrees of MPO binding [28-30].
Furthermore, MPO binding correlates with MPO killing. Bacteria showing strong MPO binding were rapidly and effectively killed when MPO was present in nanomolar quantities with about 100 micromolar (µM) H2O2 (i.e., a greater than thousandfold dilution of 3% pharmacy H2O2). Streptococci and lactobacilli show relatively weak MPO-binding, and as such, these LAB are relatively protected from MPO microbicidal action.
3.1. Hypochlorite and Microbicidal Action
In the acidic milieu of the neutrophil’s phagolysosome, or in acidic body spaces populated by LAB flora, MPO catalyzes the oxidation of chloride (Cl−) to hypochlorous acid (HOCl). Hypochlorite, the conjugate base of hypochlorous acid, is the active ingredient in household bleach, and has been employed as a disinfectant and deodorizing agent since Claude Louis Berthollet introduced it as Eau de Javel in 1789 [31-33]. Hypochlorous acid is a weak acid with a pKa of 7.5, and as such, the acid predominates in phagolysosomal and fermentation milieux.
The chloronium (Cl+) character of HOCl allows it to participate in a variety of reactions resulting in dehydrogenations and chloramine formation. The bactericidal action of hypochlorite is broad and complete at a concentration of 6 µM. However, a thousandfold higher hypochlorite concentration, i.e., 6 mM, is required for the same level of microbicidal action when human erythrocytes are added at a ratio of about five erythrocytes per bacterium [21]. Although hypochlorite shows potent microbicidal action, it lacks specificity, confirming Alexander Fleming’s comment that “leukocytes are more sensitive to the action of chemical antiseptics than are the bacteria, and, in view of this, it is unlikely that any of these antiseptics have the power of penetrating into the tissues and destroying the bacteria without first killing the tissues themselves” [34].
3.2. Reaction Radius of Singlet Oxygen
The critical importance of microbe-specific MPO binding is best understood relative to the reactive lifetime of 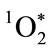 and its restricted radius of reactivity [35,36]. Like chlorine gas (Cl2),
and its restricted radius of reactivity [35,36]. Like chlorine gas (Cl2), 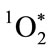 has singlet spin multiplicity, is a potent electrophilic reactant, and participates in highly exergonic reactions [18,37]. Unlike Cl2,
has singlet spin multiplicity, is a potent electrophilic reactant, and participates in highly exergonic reactions [18,37]. Unlike Cl2,  is a metastable electronically excited state with a relatively short lifetime. Singlet spin multiplicity is critically important for reactivity. Understanding the role of multiplicity in
is a metastable electronically excited state with a relatively short lifetime. Singlet spin multiplicity is critically important for reactivity. Understanding the role of multiplicity in  reactivity is best approached by considering the Wigner spin conservation rules [38,39]. In essence, the neutrophil leukocyte changes the spin quantum number of oxygen, thus removing the barrier to spin-allowed reaction with singlet multiplicity biological molecules. The wet combustive oxygenations that follow produce electronically excited oxygenation products. As shown in Figure 1, light emission or chemiluminescence (CL) is an energy product of p* → n electron relaxation of the excited carbonyl functions generated.
reactivity is best approached by considering the Wigner spin conservation rules [38,39]. In essence, the neutrophil leukocyte changes the spin quantum number of oxygen, thus removing the barrier to spin-allowed reaction with singlet multiplicity biological molecules. The wet combustive oxygenations that follow produce electronically excited oxygenation products. As shown in Figure 1, light emission or chemiluminescence (CL) is an energy product of p* → n electron relaxation of the excited carbonyl functions generated.
The short lifetime of  restricts reactivity to within a space of less than a micron from its point of generation [28,40,41]. MPO microbicidal effectiveness is limited by its proximity to the target microbe. For successful microbicidal action, primary production of HOCl, and especially, secondary production of
restricts reactivity to within a space of less than a micron from its point of generation [28,40,41]. MPO microbicidal effectiveness is limited by its proximity to the target microbe. For successful microbicidal action, primary production of HOCl, and especially, secondary production of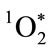 , must occur sufficiently close to the target microbe for adequate oxygenation activity. The concentration of HOCl decreases with the distance from the MPO production site. Once HOCl reacts with a second H2O2 molecule to generate
, must occur sufficiently close to the target microbe for adequate oxygenation activity. The concentration of HOCl decreases with the distance from the MPO production site. Once HOCl reacts with a second H2O2 molecule to generate , the reactive radius is restricted to within about 0.2 µm. Chloramines produced by direct reaction of HOCl with amine components of the microbe, also react with additional H2O2 to produce
, the reactive radius is restricted to within about 0.2 µm. Chloramines produced by direct reaction of HOCl with amine components of the microbe, also react with additional H2O2 to produce , and such reactions are facilitated by an acidic milieu.
, and such reactions are facilitated by an acidic milieu.
The results of Figure 2 illustrate the relationship of selective binding to selective killing. In this experiment, suspensions of Escherichia coli (~106) and S. sanguinis (~107) plus human erythrocytes (~107) were added together to yield a final ratio of about one E. coli to ten S. sanguinis and to five erythrocytes. The top plate shows the results in the absence of MPO. Note the presence of large colonies of E. coli and the absence of small S. sanguinis colonies. The middle plate shows the consequence of including 1.9 nM MPO. This small concentration of MPO significantly decreased the colonies of E. coli and also allowed the emergence of small colonies of S. sanguinis. The bottom plate shows the effects of 5.6 nM MPO. Only a single colony of E. coli developed, however, several hundred small colonies of S. sanguinis are now clearly visible.
In this experiment S. sanguinis metabolism is the only source of H2O2. E. coli are catalase positive and relatively well protected from low concentrations of H2O2. Erythrocytes show no significant MPO binding and contain abundant catalase. Introducing 107 erythrocytes with their several magnitudes greater mass than E. coli also provides competitive substrate for reaction with available
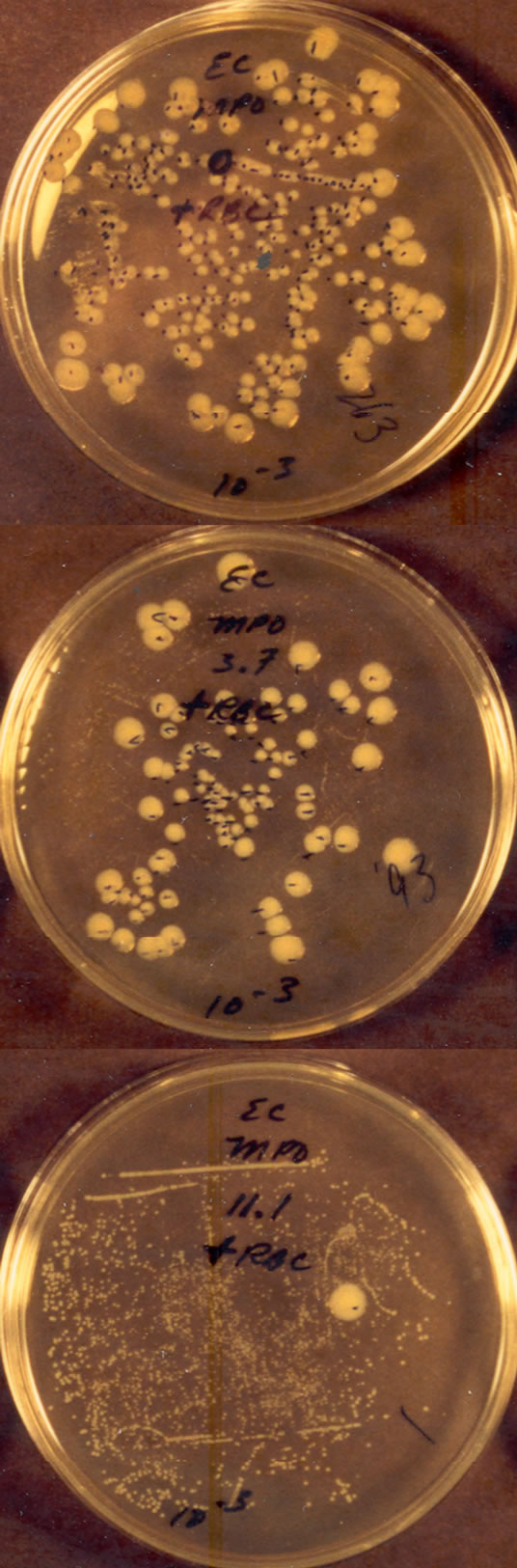
Figure 2. Effects of MPO on a co-suspension of live E. coli and S. sanguinis (about one E. coli per ten S. sanguinis) after thirty minutes exposure at 23˚C. The 10−3 CFU dilution Petri plates are shown. Human erythrocytes were present at a ratio of about five erythrocytes per E. coli. No H2O2 was added. The suspension of the top plate contained no MPO, the suspension of the middle plate contained 1.9 nM MPO, and the suspension of the bottom plate contained 5.6 nM MPO. The plates were allowed to incubate for more than a day to allow growth of the smaller S. sanguinis colonies [21].
oxidants. The absence of measurable hemolysis confirmed the absence of erythrocyte damage during the course of the experiment [21].
LAB-MPO microbicidal action in the presence of erythrocytes demonstrates the highly selective nature of MPO-mediated oxidative attack. Only the MPO-binding E. coli were killed. The absence of significant MPObinding to S. sanguinis and erythrocytes, and the proximity requirement imposed by the lifetime of 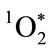 allow LAB-MPO synergistic microbicidal action against E. coli. In the presence of relatively low H2O2 concentrations and low MPO concentrations, selectivity of MPO binding results in selectivity of killing.
allow LAB-MPO synergistic microbicidal action against E. coli. In the presence of relatively low H2O2 concentrations and low MPO concentrations, selectivity of MPO binding results in selectivity of killing.
4. LAB Fermentative Metabolism and MPO
LAB are incapable of cytochrome synthesis, and conesquently, metabolism is fermentative. Nonetheless, these LAB play dominant roles in the flora of the mouth, vagina and lower gastrointestinal tract. In contradistinction to other Gram-positive and to all Gram-negative bacteria, LAB show very low MPO binding. LAB fermentative metabolism provides the acidic milieu and sufficient H2O2 for MPO microbicidal action, as demonstrated by the results shown in Figure 2, and illustrated in the schematic of Figure 3.
LAB show poor MPO binding. Thus, the proximity requirement necessary for effective MPO microbicidal action spares LAB. At low MPO concentrations LAB are protected from their metabolic product H2O2. Instead, LAB-MPO synergistic microbicidal action is focused on competing MPO-binding microbes. The presence MPO in milieux containing LAB favors the killing of microbes showing significant MPO binding. As such, LAB-MPO synergy provides a mechanism for establishing and maintaining LAB as the dominant microbes of the normal flora of man.

Figure 3. Similar roles of neutrophil metabolism and LAB metabolism in driving MPO combustive microbicidal action with chemiluminescence [17].
5. Acknowledgements
The support of ExOxEmis, Inc. is gratefully acknowledged.