Antioxidant potentials of various solvent extracts from stem bark of Enantia chlorantha ()
1. INTRODUCTION
Oxidation is one of the major causes of chemical spoilage, resulting in rancidity and/or deterioration of the nutritional quality, color, flavor, texture and safety of foods [1,2]. It is well known that reactive oxygen species (ROS) formed in vivo, such as superoxide anion, hydroxyl radical and hydrogen peroxide, are highly reactive and potentially damaging transient chemical species. The oxidative damages caused by ROS on lipids, proteins and nucleic acids may trigger various chronic diseases, such as coronary heart disease, atherosclerosis, cancer and aging [3,4]. Antioxidant refers to a compound that can delay or inhibit the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidative chain reactions and can thus prevent or repair the damage done to the body’s cells by oxygen [5]. Plants, including herbs and spices, have many phytochemicals which are a potential source of natural antioxidant, e.g., phenolic diterpenes, flavonoids, alkaloids, tannins and phenolic acids [6-8]. Natural antioxidants are known to protect cells from damage induced by oxidative stress, which is generally considered to be a cause of aging, degenerative diseases, and cancer [9]. These health promoting effects of antioxidants from plants and spices are thought to arise from their protective effects by counteracting ROS.
Enantia chlorantha commonly known as African yellow wood and also called “Awopa or Dokita Igbo Osomolu” in Yoruba land [10] is an ornamental tree up to 30m high with dense foliage and spreading crown. The stem is fluted, the bark is fissured geometrically and the outer bark is thin and dark brown. The plant is widely distributed along the coast of West and Central Africa, and it is also very common in the forest regions of Nigeria. In Nigeria, the stem bark of the plant is commonly used for the treatment of malaria and other ailments of the human body such as cough and wound [11].
The plant has been intensely studied for their antimicrobial activities [12] and antipyretic properties [13]. The efficacy and mechanisms of action of the plant have not been tested scientifically in most cases in order to justify its continuous use in traditional folk medicine. Therefore, the objective of this study was to determine the in vitro antioxidant activity of the solvents extracted material from stem bark of Enantia chlorantha.
2. MATERIALS AND METHODS
2.1. Sample Collection
Fresh stem barks of E. chlorantha were purchased in a local market, in Ado Ekiti metropolis, Nigeria. Authentication of the plant was carried out in the Department of Biology, Ekiti State University, Ado Ekiti, Nigeria.
2.2. Chemicals and Reagents
Chemicals and reagents used such as thiobarbituric acid (TBA), 1,10-phenanthroline, deoxyribose, gallic acid, Folin-Ciocalteau’s reagent were procured from SigmaAldrich, Inc., (St Louis, MO), trichloroacetic acid (TCA) was sourced from Sigma-Aldrich, Chemie GmbH (Steinheim, Germany), dinitrophenyl hydrazine (DNPH) from ACROS Organics (New Jersey, USA), hydrogen peroxide, methanol, acetic acid and FeCl3 were sourced from BDH Chemicals Ltd., (Poole, England), thiourea, CuSO4∙5H2O, H2SO4, sodium carbonate, AlCl3, potassium acetate, Tris-HCl buffer, sodium dodecyl sulphate, FeSO4, potassium ferricyanide and ferric chloride were of analytical grade while the water was glass distilled.
2.3. Solvent Extraction
The stem bark of E. chlorantha were washed under running water, cut into bits and air dried under active ventilation at room temperature for some weeks, after which the dried samples were ground to powder and kept dry in an air-tight container prior to the extraction. 3.46 kg of the sample was weighed and pulverized and subjected to cold extraction using methanol. This was intermittently stirred and filtered after 72 hours. The crude methanol filtrate was concentrated under reduced pressure at 40˚C using rotary evaporator. Certain quantity of the crude methanol extract (ME) was partitioned successively between n-hexane, chloroform, ethylacetate and water until exhaustion. The resulting solvent tractions were concentrated and freeze dried to obtain n-hexane, chloroform, ethylacetate, and aqueous fractions (HF, CF, EF, AF) respectively. These fractions were stored in containers until used.
2.4. Determination of Total Phenolic Content
The total phenol content was determined on the extracts using the method reported by Singleton et al., [14]. Appropriate dilutions of the extracts were oxidized with 2.5 mL of 10% Folin-Ciocalteau’s reagent (v/v) and neutralized by 2.0 mL of 7.5% sodium carbonate The reaction mixture was incubated for 40 minutes at 45˚C and the absorbance was measured at 765 nm in the spectrophotometer (JENWAY 6305, Barloworld Scientific, Dunmow, United Kingdom). The total phenol content in the various extracts was subsequently calculated as gallic acid equivalent using gallic acid standard curve.
2.5. Determination of Total Flavonoid Content
The total flavonoid content of both extracts was determined using a slightly modified method [15]. Briefly 0.5 mL of appropriately diluted sample was mixed with 0.5 mL methanol, 50 µL 10% AlCl3, 50 µL 1 M Potassium acetate and 1.4 mL water, and allowed to incubate at room temperature for 30 minutes. The absorbance of the reaction mixture was subsequently measured at 415 nm and the total flavonoid content was subsequently calculated using quercetin standard curve.
2.6. DPPH Free Radical Scavenging Ability
The free radical scavenging ability of the extracts against DPPH (1,1-diphenyl-2 picrylhydrazyl) free radical was evaluated as described [16]. Briefly, an appropriate dilution of the extracts (1 mL) was mixed with 1 mL of 0.4 mM methanol solution containing DPPH radicals, the mixture was left in the dark for 30 min and the absorbance was measured at 516 nm. The control was carried out using 2 mL DPPH solution without the test samples. The DPPH free radical scavenging ability was subsequently calculated.

2.7. Determination of Reducing Property
The reducing property of the extracts was determined by assessing the ability of the extract to reduce FeCl3 solution as described [17]. A 2.5 mL aliquot was mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50˚C for 20 min and then 2.5 mL of 10% trichloroacetic acid was added. This mixture was centrifuged at 650 rpm for 10 min. 5 mL of the supernatant was mixed with an equal volume of water and 1 mL of 0.1% ferric chloride. The absorbance was measured at 700 nm. The ferric reducing antioxidant property was subsequently calculated using ascorbic acid as standard.
2.8. Determination of Nitric Oxide Scavenging Activity
Nitric oxide was generated from sodium nitroprusside and measured by Griess’ reaction [18]. Sodium nitroprusside (5 mM) in standard phosphate buffer saline solution (0.025 M pH 7.4) was incubated with different concentrations (25 ‐ 200 μg/ml) of the test extract dissolved in phosphate buffer saline (0.025 M, pH 7.4) and the tubes were incubated at 25˚C for 5 hr. Control experiments without the test compounds but equivalent amounts of buffer were conducted in identical manner. After 5 hr, 0.5 ml of incubation was removed and diluted with 0.5 ml of Griess’ reagent (1% sulphanilamide, 2% Ophosphoric acid and 0.1% napthyl ethylenediamine dihydrochloride). The absorbance of the chromophore formed during diazotization of nitrite with suphanilamide and its subsequent coupling with napthyl ethylene diamine was read at 546 nm. The experiment was repeated in triplicate.
2.9. Scavenging of Hydroxyl Radical
The scavenging activity of samples in DMSO (0.85% v/v in 0.1 M phosphate buffer, pH 7.4) on the hydroxyl radical (OH●) was measured by the deoxyribose method [19] with a slight modification. The deoxyribose assay was performed in 10 mM phosphate buffer (pH 7.4) containing 2.5 mM deoxyribose, 1.5 mM H2O2, 100 μM FeCl3, 104 μM EDTA, and the test sample (0.5 mg/mL). The reaction was started by adding ascorbic acid to a final concentration of 100 μM. The reaction mixture was incubated for 1 h at 37˚C in a water-bath. After incubation, the color was developed by addition of 0.5% thiobarbituric acid followed by ice-cold 2.8% trichloroacetic acid in 25 mM NaOH and heating for 30 min at 80˚C. A control was performed without samples (A1). The sample (A2) was cooled on ice and the absorbance was measured at 532 nm. The hydroxyl radical scavenging activity (HRSA) was calculated by the following equation: HRSA% = (A1 − A2/A1) × 100.
2.10. Lipid Peroxidation Assay
Experimental Animals
Male Wistar albino rats weighing between 190 and 250 g were purchased from the Central Animal House, Department of Biochemistry, University of Ilorin, Ilorin, Nigeria. They were housed in stainless steel cages under controlled conditions of a 12 hours light/dark cycle, 50% humidity, and 28˚C temperature. The rats were allowed asses to food and water ad libitum. The animals were used in accordance with the procedure approved by the Animal Ethics Committee of the Ekiti State University, Ado Ekiti, Nigeria.
2.11. Preparation of Tissue Homogenates
The rats were decapitated under mild diethyl ether anaesthesia and the liver (tissue) was rapidly dissected and placed on ice and weighed. This tissue was subsequently homogenized in cold saline (1/10 w/v) with about 10 up-and-down strokes at approximately 1200 rev/min in a Teflon glass homogenizer (Mexxcare, mc14 362, Aayushi Design Pvt. Ltd., India). The homogenate was centrifuged (KX3400C Kenxin Intl. Co. Hong Kong) for 10 minutes at 3000 × g to yield a pellet that was discarded, and a low-speed supernatant (SI), which was kept for lipid peroxidation assay [20].
2.12. Lipid Peroxidation and Thiobarbibutric Acid Reactions
The lipid peroxidation assay was carried out using the modified method of Ohkawa et al. [21]. Briefly 100 µL of the SI fraction was mixed with a reaction mixture containing 30 µL of 0.1 M pH 7.4 Tris-HCl buffer, extract (100 µL) and 30 µL of 25 µM freshly prepared FeSO4. The volume was made up to 300 µL by water before incubation at 37˚C for 2 hours. The colour reaction was developed by adding 300 µL 8.1% SDS (Sodium dodecyl sulphate) to the reaction mixture containing SI, this was subsequently followed by the addition of 600 µL of acetic acid/HCl (pH 3.4) mixture and 600 µL of 0.8% Thiobarbituric acid (TBA). This mixture was incubated at 100˚C for 1 hour. Thiobarbituric acid reactive species (TBARS) produced were measured at 532 nm and expressed as Malondialdehyde (MDA) produced (% control) using Malondialdehyde standard curve (0 - 0.035 mM).
2.13. Data Analysis
The results of the replicates were pooled and expressed as mean ± standard deviation. Analysis of variance and Student’s t-test were carried out [22]. Significance was accepted at P < 0.05.
3. RESULTS AND DISCUSSION
3.1. Total Phenolic Content
The anti‐oxidant activity of methanol extract (ME), nhexane, chloroform, ethylacetate, and aqueous fractions (HF, CF, EF and AF respectively) of stem bark of E. chlorantha were measured by different methods like DPPH scavenging activity, ferric reducing property (FRAP), nitric oxide radical scavenging activity and hydroxyl radical scavenging activity.
Phenolic compounds can protect the human body from free radicals, whose formation is associated with the normal metabolism of aerobic cells. They are strong antioxidants capable of removing free radicals, chelate metal catalysts, activate antioxidant enzymes, reduce atocopherol radicals and inhibit oxidases [23].
The findings of our study revealed high concentration of total phenolics in all the solvent extracts of stem bark of E. chlorantha (Table 1). Methanol fraction had the highest total phenolic content (838.03 mg/g), while hexane fraction had the lowest value (118.03 mg/g). The total phenolic content in decreasing order was methanol fraction > aqueous fraction > ethylacetate fraction > chloroform fraction > hexane fraction (P < 0.05). However, the values obtained are higher than what was reported for some hot peppers [24] and green teas [25]. It is known that the higher the phenolic content of plant, the higher its antioxidant activities. Rice-Evans et al. [25] reported that the antioxidant properties of phenolic acids are due to their redox properties, ability to chelate metals and quenching of singlet oxygen.
3.2. Total Flavonoid Content
Furthermore, the total flavonoid content expressed as quercetin equivalent of all the solvent extracts was significantly high (Table 2). The concentration was appreciably high in chloroform fraction followed by methanol extract, aqueous and ethylacetate fractions while hexane fraction had the lowest. Generally, n-hexane fraction had the lowest total phenolic and flavonoid contents. The appreciable level of total phenolic and flavonoid contents in the extracts of the stem bark might be responsible for the use of this plant for the treatment of radical related problems such as malaria and cough [26].
3.3. DPPH Radical Scavenging Assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) is widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors, and to evaluate antioxidant activity of foods [27]. The trend of inhibition of DPPH radical by the extracts was concentration dependent (Table 3). At the highest concentration (800 µg/ml), methanol extract exhibited the highest inhibition of DPPH radical which corresponds to its phenolic contents. This was followed by aqueous, ethylacetate, chloroform fractions whereas, n-hexane fraction showed the least inhibition. The DPPH radical scavenging ability of the extracts can be ranked in the order methanol extract (96.53%) > aqueous fraction (89.18%) > ethylacetate fraction (74.29%) > chloroform fraction (68.16%) > hexane fraction (52.24%). The observed differential scavenging activities of the extracts against the DPPH system could be due to the presence of different compounds in the extract. Although the DPPH radical scavenging activities of the plant were greater (P < 0.05) than that of ascorbic acid used as a reference drug, the strong inhibition displayed on DPPH radical could be linked to polyphenolic compounds which are capable of donating electrons to neutralize free radicals and thus, could be a promising therapeutic agent to treat stress induced pathological conditions.
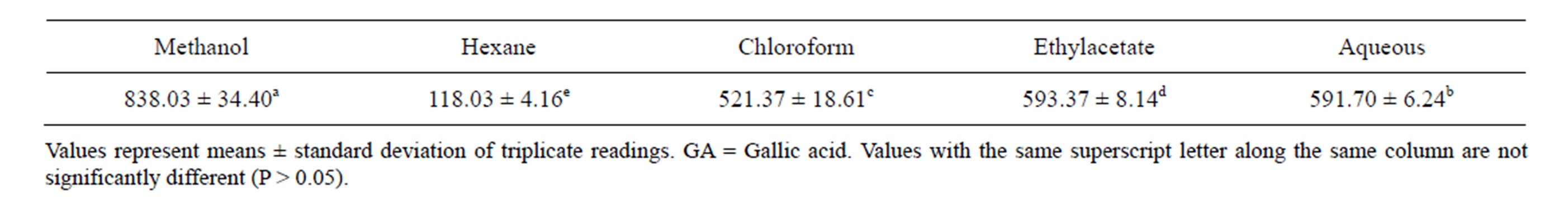
Table 1. Total Phenolic content of various extracts of stem bark of Enantia chloranta (mg GA/g).

Table 2. Total flavonoid content of various extracts of stem bark of Enantia chloranta (mg QE/g).

Table 3. DPPH radical scavenging ability of various extracts of stem bark of Enantia chloranta (%).
3.4. Ferric Reducing Ability
Furthermore, the reducing power of the extractable phytochemicals from E. chlorantha expressed as ascorbic acid equivalent (AAE) is presented in Table 4. The trend of the reducing capacity of the extracts was concentration dependent (Table 4). At a concentration of 800 µg/ml, the result revealed that methanolic extract of stem bark of E. chlorantha had a significant (P < 0.05) higher reducing power followed by aqueous and chloroform fractions while hexane and ethylacetate fractions had the least. The reducing power as typified by the ability of the plant extracts to reduce Fe3+ to Fe2+ is a potent antioxidation defense mechanism, and two mechanisms available to effect this reducing power is by electron transfer and hydrogen atom transfer [28]. Allhorn et al. [29] reported that the reducing property can be a novel antioxidation defense mechanism, possibly through the ability of the antioxidant compound to reduce transition metals.
3.5. Nitric Oxide Scavenging Assay
Nitric oxide (NO) radical is generated from sodium nitroprusside at physiological pH. It is a highly reactive compound that is capable of changing the structural and functional behavior of many cellular components [30]. The extracts of stem bark of Enantia chloranta inhibited nitric oxide radical in a concentration dependent manner (Table 5). At a concentration of 200 µg/ml, the percentage inhibition of nitric oxide radical was maximum for methanolic extract with 69.04%.
The nitric oxide radical scavenging activity in decreasing order was methanol extract (69.04%) > aqueous fraction (52.24%) > chloroform fraction (48.59%) > ethylacetate fraction (32.14%) > n-hexane fraction (20.09%). Nitric oxide is free radical produced in biological cells, involved in regulation of various physiological processes. Nitric oxide is very unstable species under aerobic conditions. It reacts with oxygen to produce stable product nitrate and nitrite through intermediates NO2, N2O4 and N3O4. In present study, the inhibitory potentials of the extracts against NO radical can be attributed to their ability to compete with oxygen and its derivatives [31].
3.6. Hydroxyl Radical Scavenging Assay
Among the reactive oxygen species (ROS), hydroxyl radicals are the most reactive and redominant radicals generated endogenously during aerobic metabolism to initiate cell damage in vivo [32]. We examined the inhibitory action of the extracts on deoxyribose degradation which gives an indication of hydroxyl radical scavenging action [33,34]. Hydroxyl radical scavenging activities of E. chlorantha extracts at a concentration of 0.5 mg/mL in relation to ascorbic acid at the same concentration are shown in Table 6. The hydroxyl radical scavenging activity increased with increasing concentration of the extracts (Table 6). At a concentration of 40 µg/ml, the result in decreasing order of hydroxyl radical scavenging activity was ethylacetate (86.30%) > methanol extract (84.30%) > chloroform (77.96%) > aqueous (72.75%) > n-hexane fractions (66.13%). Hydroxyl radical scavenging activity of the rthylacetate fraction was higher than other extract and fractions used (Table 6).
3.7. Inhibition of Lipid Peroxidation
Furthermore, the incubation of the liver tissue homogenate in the presence of FeSO4 caused a significant increase in the malondialdehyde (MDA) contents of the liver (Table 7). However, the various solvent extracts of

Table 4. Ferric reducing antioxidant property (FRAP in %) of various extracts of stem bark of Enantia chloranta.
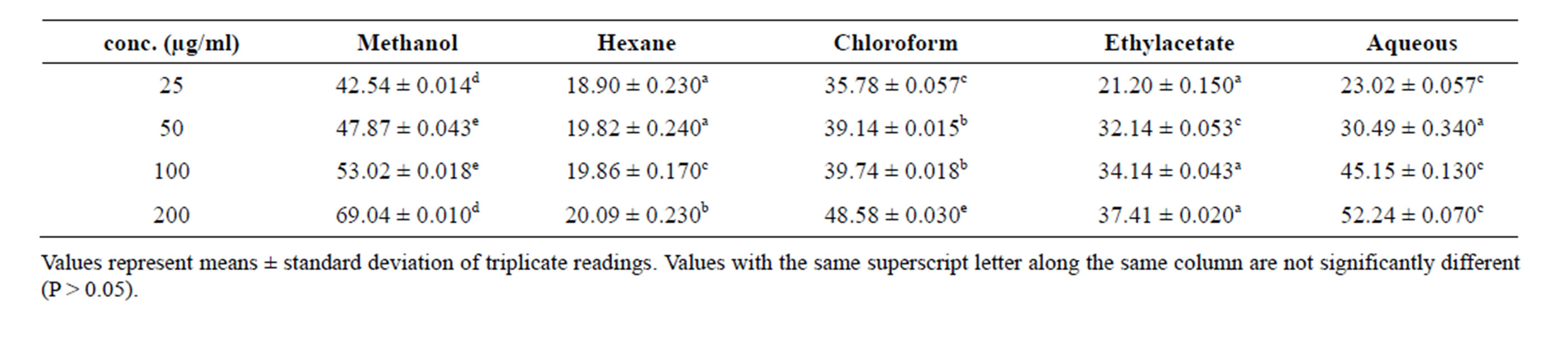
Table 5. Nitric oxide radical scavenging activity of various extracts of stem bark of Enantia chloranta.

Table 6. Hydroxyl radical scavenging activity of various extracts of stem bark of Enantia chloranta.

Table 7. Percentage inhibition of Fe2+-induced lipid peroxidation in rat liver by various extracts of stem bark of Enantia chloranta (%).
the stem bark of Enantia chlorantha showed a significant decrease in the MDA contents of the liver in a dose-dependent manner; ME had a significant (P < 0.05) higher inhibitory effect (75.2% at 20 µg/ml) on Fe2+-induced lipid peroxidation in the rat liver homogenates than other fractions. However, the CF showed the overall highest inhibitory percentage at the lowest concentration. The order of inhibition was ME > CF > HF >AF > EF. Even though, vitamin C showed better inhibition against lipid oxidation than the plant extracts (Table 7), the inhibition of lipid peroxidation by the extracts can be adduced to the presence of phenolic compounds. The decrease in the Fe2+ induced lipid peroxidation in the rat liver homogenates in the presence of the extracts could be as result of the ability of the extracts to chelate Fe2+ and/or scavenge free radicals produced by the Fe2+ catalyzed production of reactive oxygen species (ROS) in the rat liver.
4. CONCLUSION
This study revealed that the stem bark of E. chlorantha contains appreciable amounts of phenolic and flavonoid contents that are capable of eliciting potent antioxidant activities. The antioxidant profile of this plant can be harnessed to treat radical related pathological conditions. The mechanism of antioxidant action was based on the ability of its extracts to donate electrons, reduce ferric ions, scavenge nitric oxide, DPPH and hydroxyl radicals. This has justified the use of this plant in folk medicine for the treatment of stress related diseases. The antioxidative potential of the plant was dependent on the solvent of extraction which means that methanol may be a good solvent while hexane may not be a good solvent of extraction in the exploitation of the antioxidant property of E. chlorantha stem bark. Thus this study gives support for expanding future investigations of pharmacological activities associated with free radicals and characterization of potent extract for its main active constituents.
NOTES