Tracking Microorganisms in Production and Sale Operations of Spiced Geese ()
1. Introduction
China is the largest country with geese producing and consuming [1]. 2010 Statistical data (FAO) shown that, about 321.90 millions of geese amount of livestock on hand in China, 89.92% of the world’s total amount of livestock on hand (about 357.98 millions); 601.65 millions geese were Slaughtered in China, 94.32% of the world’s total amount of slaughtering (637.89 millions); 240.72 ten thousand tons geese meat were produced in China, 95.47% of the world’s total amount of geese meat (252.14 ten thousand tons).
Market for geese is growing, its production and consumption have increased 65.53 ten thousand tons since 2000 (FAO). Clearly, the continued growth and prosperity of the geese industry will depend, to a large degree, on its ability to supply the consumer with wholesome and safe products [2].
Spiced goose meat was one of the favorite spiced meat to Chinese because of the delicious taste and abundant nutrition [3].
In general case, traditional spiced geese meat were made in household workshop, sold in the retail stalls directly and without any microbial prevention or control measures during the whole process, such as packaging and sterilization, so microbiological safety can’t be safeguard [4].
Even though the nutritional value of geese meat is well documented, very little information is available worldwide on the microbiological aspects, especially of the spiced geese meat and the production process. The purpose of the present study was to examine the number and types of microorganisms associated with spiced geese meat and the production process. These findings may provide information that will be useful in controlling contamination during processing and extending the shelf life of spiced geese meat [5].
2. Materials and Methods
2.1. Samples
All spiced geese meat samples, airborne microorganisms samples and environmental contact substances samples were collected from a famous household workshop in Rongchang county Chongqing (China) and one of the retail outlets.
2.2. Spiced Geese Meat Sampling
According to the production process and the HACCP analysis, cooling, transportation and sale process should be the critical points of microbial contamination. So time points when products at the 5th and the 30th minute in cooling process, when products in transport process, when products at the 0th h, 1st h, 2nd h, 3rd h, 4th h in the retail stores were selected as the sampling time, corresponding samples were named C5 min, C30 min, T, S0 h, S1 h, S2 h, S3 h, S4 h.
Three parallel samples were collected at each sampling time, each sample was placed in a separate sterile plastic bag and transported to the laboratory immediately for analysis.
A 1g sample of each obtained spiced geese meat was taken aseptically by scalpel excision and placed in a sterile conical flask with 9 ml sterile saline as bacterial suspension. Decimal dilutions were carried out using the same diluents with pure water [6].
2.3. Airborne Microorganisms Sampling
Airborne microorganisms in the household workshop, transport vehicle and retail outlet were collected.
Natural sedimentation method has been used for the collection of air microorganism samples according to the national standard of the People’s Republic of China GB/ T18204.1-2000. In order to make air flow minimized, points where keep more than 1meters distance away from wall and far away from the vents were selected. Then culture dishes (diameter 9 centimeters) with different special medium were exposed 5 minutes on the sample points. Three parallel samples were collected on each sampling point, then transported to the laboratory immediately [7]. Plates were incubated at 37˚C for 48 hours, colony forming units (CFU) on the plates were counted and total aerobic counts per 1 m3 was determined according the following formula.
Total aerobic counts (CFU/m3) 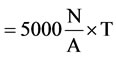
A: represent area of the culture dish (cm2);
T: represent culture dish exposure time (min);
N: represent mean colony content (CFU).
2.4. Environmental Contact Substances Sampling
Microbial samples on cooling platform, stainless steel barrel, pallet, chopping block, chopper were collected.
Sampling plane with 100 cm2 was selected randomly on surface of each contact substances. Four sterile cotton balls were used to wipe the sampling plane, then the cotton balls were put into a sterile conical flask with 20 ml sterile saline as bacterial suspension. Three parallel samples were collected of each sampling plane, then transported to the laboratory immediately after collection.
A 200 μl sample of each 20 ml bacterial suspension was taken aseptically, decimal dilutions were carried out using the same diluents with pure water [8].
2.5. Microbiological Identification and Enumeration
Bacteria were enumerated on six different media. Total aerobic counts were determined using Nutrient agar (Hangzhou Microbial Reagent CO., Ltd.) spread plates incubated at 37˚C for 48 hours [2]; Escherichia coli (E. coli) and Salmonella were determined using Maconkey agar (Beijing Aobox Biotechnology Co., Ltd.) spread plates incubated at 37˚C for 48 hours (AduGyamfi1, Torgby-Tetteh&Appiah, 2012); Fungus was determined by the spread plate method using improved Martin medium (Hangzhou Microbial Reagent Co., Ltd.); Lactic acid bacteria was determined using MRS agar (Beijing Aobox Biotechnology Co., Ltd.) spread plates incubated at 37˚C for 48 hours; Staphylococcus aureus (S. aureus) was determined by the spread plate method using BairdParker agar base. The plates were incubated at 37˚C for 48 h [9]. In order to determine S. aureus counts, random isolates from suitable plates were picked, purified and tested for electron microscopy, gram stain [10], Mannitol fermentation and catalase activity [11]; Janthinobacterium was determined using medium with peptone 20 g, potassium dihydrogenphosphate 1.5 g, magnesium sulfate 1.5 g, agar medium 15 g, distilled water 1000 ml, pH: 6.9 - 7.1, spread plates incubated at 37˚C for 48 hours. In order to determine Janthinobacterium counts, random isolates from suitable plates were picked, purified and tested for gram stain [10], electron microscopy, urea decomposition, starch hydrolysis, hydrogen sulfide production, lactose hydrolysis, sucrose hydrolysis and indole production. Furthermore, PCR/RFLP marker of Janthinobacterium was cloned and 16S rDNA was sequenced.
2.6. Statistical Analysis
For spiced geese meat sample, total aerobic counts were transformed to log10CFU/g. For Airborne microorganisms counts, the data were transformed to log10 CFU/m3. For environmental contact substance counts, the data were also transformed to log10CFU/cm2 so as to enable a true comparison of the different counts reported by other authors with that determined in this study [12].
One-Way Analysis of Variance (ANOVA) was performed, and if the ANOVA detected significant differences in group means, the Duncan Multiple Comparisons Test was used to determine which treatment groups differed significantly. All significant differences were determined at p < 0.05.
Analysis was carried out using the “SAS 8. 2” software package (SAS Institute Inc., Cary, NC, USA) for Windows XP.
3. Results and Discussion
3.1. Microorganism Identification
Special mediums were used to identify the microorganisms isolated from different samples, furthermore methods of gram stain and electron microscopy were also used, results were shown in Figure 1. The preliminary identification results indicated that E. coli, Yeast, Mildew, Lactic acid bacteria, S. aureus, Janthinobacterium were the contamination microorganisms.
In order to make sure the results correct, biochemical identification was carried out on S. aureus and Janthinobacterium, the results (Table 1) indicated that, biochemical identification results of S. aureus and Janthinobacterium were agreed with the reference answers. In order to verify the results, method of DNA sequencing was carried out, the PCR fragments of Janthinobacterium were shown in Figure 2. DNA sequence was obtained and compared to the Basic Local Alignment Search Tool (BLAST), the result shown that the obtained DNA sequence has 99% similarity with Janthinobacterium.
3.2. Microbial Quality of Geese Meat Samples
Microbial loads of spiced geese meat samples were given in Table 2, the total aerobic counts for C5 min, C30 min, T, S0 h, S1 h, S2 h, S3 h, S4 h were 2.68, 3.04, 3.16, 3.54, 4.07, 3.95, 4.21 and 4.86 log10CFU/g respectively; The mean total E. coli, Yeast, Mildew, Lactic acid bacteria, S.
 (a)
(a) (b)
(b)
Figure 1. Gram stain andelectron microscopy results of S. aureusand Janthinobacterium. (a) Staphylococcus aureu; (b) Janthinobacterium.

Figure 2. 16S rDNA fragments of agarose gel electrophoresis of Janthinobacterium.

Table 1. Biochemical identification results of S. aureus and Janthinobacterium.
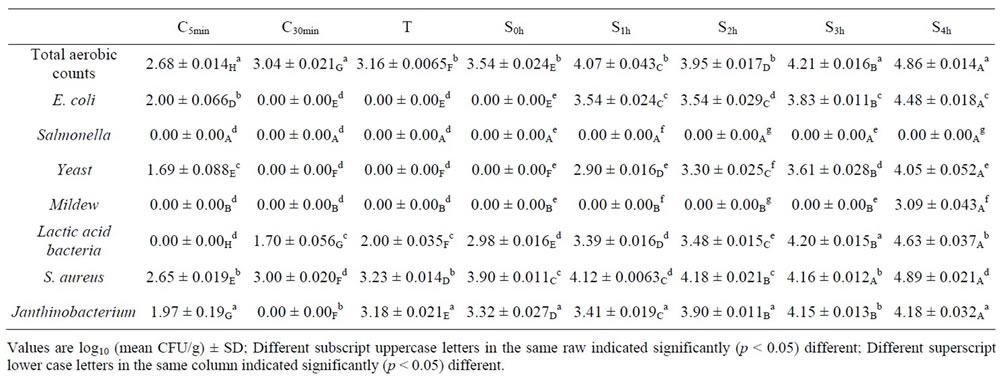
Table 2. The results of contaminated microorganism on spiced goose meat with different sampling time. Unit: log10CFU/g.
aureus, Janthinobacterium count for C5 min, C30 min, T, S0 h, S1 h, S2 h, S3 h, S4 h were 2.00, 0, 0, 0, 3.54, 3.54, 3.83 and 4.48 log10CFU/g; 1.69, 0, 0, 0, 2.90, 3.30, 3.61 and 4.05 log10CFU/g; 1.69, 0, 0, 0, 2.90, 3.30, 3.61 and 4.05 log10CFU/g; 0, 1.70, 2.00, 2.98, 3.39, 3.48, 4.20 and 4.63 log10CFU/g; 1.97, 0, 3.18, 3.32, 3.41, 3.90, 4.15 and 4.18 log10CFU/g; 2.65, 3.00, 3.23, 3.90, 4.12, 4.18, 4.16 and 4.89 log10CFU/g respectively; Salmonella not detected on each samples.
Table 2 indicated that total aerobic counts was significant (p < 0.05) increased of each spiced geese meat sample. Lactic acid bacteria, S. aureus and Janthinobacterium were detected in each processing operations and the total aerobic counts of each was increased or significant (p < 0.05) increased; E. coli, Yeast and Mildew were detected on samples entered into the retail outlet mainly and the total aerobic counts of each was increased or significant (p < 0.05) increased also.