1. Introduction
The 80% - 85% of Lung cancer is non-small-cell (NSCLC) and constitutes the leading cause of cancer-related death in both men and women in the western world [1]. For example, in 2008, 215,020 new cases are expected and 161,840 persons are projected to die from the disease in the United States [2].
Locally advanced non-small cell lung cancer (NSCLC) is a systemic disease requiring a multimodality approach for optimal treatment. Several combined modality treatments have been investigated to improve outcome in localized NSCLC. These might include local treatment, systemic before local treatment, concomitant systemic and local treatments, and systemic after local treatment. It has been suggested that preoperative chemotherapy might have a greater effect in stages I and II of the disease. In locally advanced disease, chemotherapy followed by radiotherapy was shown to increase survival when compared with radiotherapy alone. Studies comparing concurrent chemoradiation with radiotherapy only were in favor of the concomitant schedule, which improved local control. Some results have been reported with chemoradiation followed by surgery in stage IIIa and even stage IIIb disease [3].
Survival rate and treatment options can vary from stage to stage of the disease. An adequate diagnosis and staging, is necessary for personalized treatment strategies that can be chosen to gain best prognosis and less expensive. The recommended therapy [4] for stage I disease is surgical resection, with stereotactic body radiation therapy (SBRT) [5,6] reserved for those who are medically inoperable. Stage II disease is also treated with surgery followed by adjuvant chemotherapy to prevent disease recurrence. Stage IIIA disease has multiple treatment options determined by the extent of regional (nodal) involvement. Stage IIIA disease is often treated with concurrent chemotherapy and radiation, adding surgical resection (trimodality therapy) for those who are medically fit and have responded well to initial concurrent therapy. Stage IIIB disease is treated with concurrent chemotherapy and radiation. Stage IV disease is treated with systemic therapy, chemotherapy, and/or molecular targeted agents, in addition to radiotherapy.
Advanced-stage NSCLC is currently considered an incurable disease for which standard chemotherapy provides marginal improvement in overall survival at the expense of substantial morbidity and mortality. Furthermore, less than 30% of patients with metastatic NSCLC have a response to platinum-based chemotherapy, the most commonly used initial treatment in this stage of the disease. Even with the addition of newer agents, such as bevacizumab, to chemotherapy, the median overall survival of patients with metastatic NSCLC remains approximately 1 year [7,8], and only 3.5% of patients with metastatic NSCLC survive 5 years after diagnosis [9].
Recent improvements in systemic therapy are the new treatment paradigms with maintenance chemotherapy [10] and the use of targeted therapies in tumours selected by molecular biological characteristics [11]. Another innovative strategy that may improve overall survival (OS), with reduced toxicity compared with conventional cytotoxic chemotherapy, is the use of therapeutic cancer vaccines.
The signaling pathway of the epidermal growth factor receptor (EGFR), a cell-surface receptor, is activated in more than half of patients with NSCLC, and this activation can be the result of protein overexpression, increased gene copy number, or genetic mutations [12,13].
The relationship between the system formed by the Epidermal Growth Factor receptor (EGFR) and its ligands with cancer development is well known. In epidermoid origin tumors, there is an over-expression of the EGFR that relates to bad prognoses and early relapses after surgery. That is why this system has become an important target for anti-tumor therapies.
Cell proliferation mechanisms are initiated with the binding of EGF to EGFR. Our therapeutic approach consists of a vaccine with an EGF formulation making it immunogenic and inducing a humoral immune response. The production of specific anti-EGF antibodies that bind to the autologous EGF, prevents it from binding to the EGFR thereby triggering the cell proliferation mechanisms derived from that interaction [14-17].
Cancer is the second cause of death in Cuba, and there is currently a clear increase in the number of deaths caused by this disease: in 2010, the death rate was 197.5 per 100,000 habitants, against 190.4 in 2009. Cancer is also the primary cause of years of potential life lost (an estimate of the average years a person would have lived if he had not died prematurely or a measure of premature mortality), with 34.5 per 1000 inhabitants. In particular, lung cancer is one of the diseases with the highest incidence, and the first cause of death among patients with tumors in Cuba, with more than 3000 deaths in 2010. Cancer therefore constitutes a major public health problem. The Cuban Ministry of Public Health has thus implemented a comprehensive Cancer control program, operating across all levels of the national public health system. This program constitutes a new therapeutic approach to the disease, with biotechnology serving as a bridge between basic immunology research and public health. The Center of Molecular Immunology (CIM) is one of the centers of the Scientific Pole in Cuba devoted to research, development, and manufacturing of human biotechnological products.
The Epidermal Growth Factor Receptor (EGFR) is a well-known oncogene. Its over activation can induce malignant transformation of a normal cell, signaling inhibition of apoptosis, cell proliferation, angiogenesis, metastasis and tumor-induced proinflammatory, and immunosuppressive processes. The EGFR signaling and transduction pathway can be efficiently interrupted by EGF deprivation, direct specific mAb receptor inhibition, or low molecular weight molecules competing intracellularly with adenosine triphospate (ATP) for the recaptor’s tyrosine kinase activity site, with negative repercussions on cell proliferation and, consequently, on tumor development [18,19].
CIMAvax®EGF is a registered vaccine for the treatment of non-small cell lung cancer (NSCLC) patients and it has undergone five phases I/II, one phase II, and two phases III clinical trials. The results of these clinical trials led the Cuban regulatory authority (CECMED) to register this therapeutic vaccine for use in adult patients with stage IIIB/IV non-small-cell lung cancer.
The objective of the study is to evaluate some risk factors of death using survival methods. The Cox proportional hazards regression model for censored survival data [20] is widely used to identify the factors that influence the overall survival, among a set of potential risk factors. One appeal of this model is its semi-parametric form (the model is specified up to an unknown function, called the baseline hazard function, and a finite number of unknown regression parameters), which provides a flexible framework for data analysis. Moreover, this model is easily implemented from any modern statistical software. However, this model heavily relies on the assumption that the risk factors, or covariates, have a proportional effect over time. This is the so-called proportional hazards assumption. Precisely, this assumption states that the hazards ratio of two individuals is constant over time, which is likely to be too restrictive in practice [21]. Assessing the proportional hazards assumption is therefore a crucial preliminary step when applying a Cox regression model. This can be achieved by using specific graphical procedures (such as log-cumulative hazards plots, Schoenfeld residual plots, …) and goodness-of-fit tests (tests that include time-dependent covariates, tests based on generalizations of the Cox model, …) [21-28].
Once a deviation from the proportional hazards assumption has been identified (such as for the variable age in our setting), one simple alternative to the Cox model is the so-called stratified Cox model, which accommodates non-proportional hazards for a continuous covariate by: 1) discretizing this covariate and 2) assuming distinct baseline hazard functions within each of the modalities (also called strata) of the discretized variable. The stratified Cox model includes the variables satisfying the proportional hazards assumption as covariates, while the variable used to stratify is not included in the regression component [28,29]. The stratified Cox model can be fitted using the same techniques as the usual Cox model (such as the partial likelihood), and all the nice Cox model modeling and inferential tools (time-dependent covariates, likelihood ratio tests, model selection) can be generalized to the stratified model [30,31].
In this paper, a stratified Cox regression model (with age used as the stratifying variable) is fitted to data arising from two clinical trials designed to evaluate the effects of various risk factors on the overall survival of patients with non-small cell lung. The two clinical trials were conducted by our center (CIM), in cooperation with the Cuban National Coordinating Center of Clinical trials (CENCEC).
Purpose
The aim of this work was to evaluate the effects of several potential prognostic factors on the overall survival of patients with non-small cell lung cancer, treated with the CIMAvax®EGF vaccine.
2. Patients and Methods
2.1. Study Design and Treatment
Our analysis is based on data collected in two clinical trials. One is a finished phase II trial including 80 patients (under a balanced design), and the other is an on-going phase III trial including (for the time being) 283 patients (under an unbalanced design 1:2). Both trials are controlled, with two treatment arms: one arm received the CIMAvax®EGF vaccine and the other received the standard treatment for this condition (platinum-based combination chemotherapy). In both trials, the duration from the inclusion to the death (referred to as the overall survival in the sequel) was defined as the primary endpoint. Some demographic and disease-related variables were also measured in both studies: age, sex, clinical stage of cancer, performance status (0: Fully active, able to carry on all pre-disease performance without restriction, 1: Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work be more precise, 2: Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours), histological type (1: adenocarcinoma, 2: non-adenocarcinoma (squamous cell lung carcinoma, or large cell lung carcinoma)). Both studies were stratified according to whether the patients were under or over 60 years of age. Some additional variables were measured but their analysis falls beyond the scope of the present paper. These variables include: the response rate (1: complete response, 2: partial response, 3: stable disease, 4: progression disease), objective response (1: complete response + partial response, 2: stable disease + progression disease) and control disease (1: complete response + partial response + stable disease, 2: progression disease), Toxicity and Quality of Life as a secondary variables.
The ethics boards of all institutions involved in these trials approved the protocols, and all patients provided written informed consent. The data were collected, managed, and analyzed at CIM and CENCEC. The present work focuses on the evaluation of prognostic factors influencing the overall survival of the patients included in the two trials.
2.2. Eligibility Criteria
Patients had histologic or cytological evidence of NSLC (Adenocarcinome and Non Adenocarcinome), ECOG performance status 0, 1, or 2, stage IIIB and IV, and adequate hematologic, renal, and hepatic function.
2.3. Statistical Analysis
Comprehensive descriptive statistical analyses were first conducted, in order to characterize the study population of lung cancer cases. Then, age-stratified Cox regression analyses were performed on all randomly assigned patients as per the intent-to-treat principle. Independent stratified Cox regression models were first fitted to each of the trials. A stratified Cox model was then fitted to the combined data. All these analysis included the following explanatory variables: sex, clinical stage of cancer, performance status and histological type. In all three analyses, a backward selection procedure was used to remove the covariates that did not influence the overall survival, and to achieve the best fit.
3. Results and Discussion
3.1. Patient Characteristics
For the first trial (May, 2002-December, 2005), 80 patients were randomly assigned (40 to CIMAvax®EGF and 40 to a control group) to one of six centers located in Cuba. The second trial has recruited 283 patients (Mar, 2006-December, 2011) (199 to CIMAvax®EGF and 84 to the control group in 14 hospitals in Cuba. Baseline characteristics were well-balanced between the arms, and all assigned patients were included in intent-to-treat analyses. The demographic characteristics of all the patients are summarized in the Table 1.
3.2. Prognostic Factors Evaluation
The results of the stratified Cox regression analyses of each trial and of the combined data are summarized in Tables 2-4 respectively. As mentioned above, the data were stratified according to whether the patients were under or over 60 years of age.
First, from the Table 2 (results from the Phase II trial), it appears that none of the covariates investigated here is statistically significant, at the level 5%. Thus, none of these covariates seems to influence the overall survival. As a complementary analysis, we obtained a forest plot. Forest plots have become a useful graphical method for displaying treatment effects across subgroups. Typically, a forest plot presents an overall effect (for all randomized participants) and then various subgroup computations on a common axis. Each point plotted represents a comparison between treatment and control participants in the relevant subgroup and is accompanied by its 95%CI.
Forest plots show the overall estimate and confidence intervals (in each subgroups) indicate which direction favours treatment or control. Subgroup estimate are displayed underneath the overall estimate.
In Figure 1 we observe the no adenocarcinoma, performance status 0-1, stage IV, age less than 60 years and male patients are more benefit in the experimental group.
In Table 3, the results from the analysis of phase III data are displayed. From the p values, in the older stratum none of the prognostic factors seem to be non-significative, while for younger patients stage of tumour IV is found to be important prognostic factor which affect the survival and patients with this characteristic benefit more if they receive CIMAvax®EGF. Patients receiving the control treatment who tumours are Stage IV have 4.6 times the hazard faced by patients in the vaccine group. In Figure 2 we observe the same results as those observed for phase II study.
According to the data of combined phase II and III, it is observed in Table 4 that PS equal to 0 or 1, stage IV and male sex influence the overall survival time in younger patients. For PS 0 - 1 the risk of death for people in control group is 1.4 the risk of those that were vaccinated. For Stage IV this risk is 3.2 and for male patients is 1.5. This shows a clear benefit for those patients that received CIMAvax®EGF.
In Figure 3, the lines suggest that patients with PS 0 or 1, stage IV and age under 60 years may benefit from CIMAvax®EGF vaccine treatment, this will allow to design further studies to look for evidence for the this group of patients. There is clear evidence that for patients with Stage IV the vaccine increase the survival rate, since the heterogeneity test is significative.

Table 2. Results of a stratified Cox regression analysis of the phase II trial.
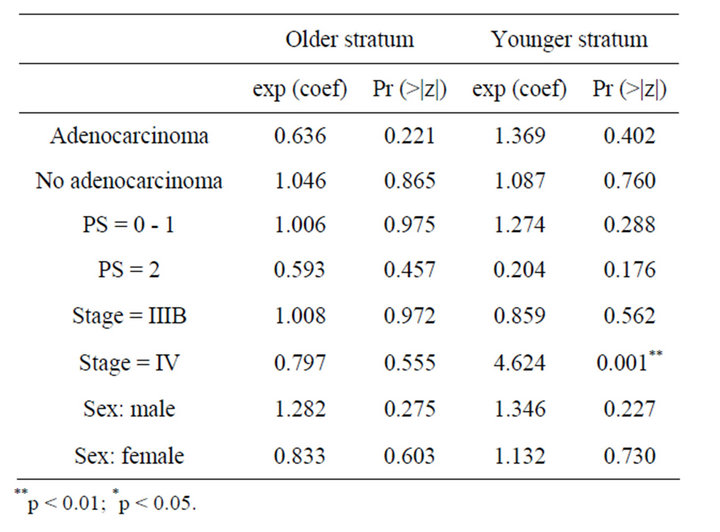
Table 3. Result of Cox regression models for phase III trial.

Table 4. Result of Cox regression models for combined phase II & III trial.

Figure 1. Forest plot for phase II trial.

Figure 2. Forest plot for phase III trial.

Figure 3. Forest plot for combined phase II & phase III trial.
4. Conclusion
In this paper, we have used stratified Cox regression analysis to evaluate the effects of some potential prognostic factors on the overall survival of patients with nonsmall cell lung cancer, treated with the CIMAvax®EGF vaccine. Stratifying over age was necessary because of the violation of the proportional hazards assumption for this variable. According to the results of phase II trial and phase III and for the combined data we found that the vaccinated group has a better performance if the patients have a performance status 0 or1, stage IV and age under 60 years. These prognostic factors influence overall survival in a positive way for those patients that received CIMAvax®EGF.
5. Acknowledgements
This work has been supported by a UICC International Cancer Technology Transfer Fellowship.
NOTES
#Corresponding author.