High Osteoconductive Surface of Pure Titanium by Hydrothermal Treatment ()
1. Introduction
Nowadays, titanium (Ti) has been successful for implant applications especially in dental and orthopedic fields because of its superior properties such as good biocompatibility, good ductility, high fatigue and tensile strengths, lower allergenicity and high corrosion resistance arising from the formation of a self-healing passive oxide layer on their surface. Many studies about the long-term success rates of Ti implants have been well reported [1,2]. However, it cannot bond with bone directly and contribute new bone formation on its surface at the early stage after implantation due to insufficient bioactivity [3].
In many in vitro tests, the surface properties of Ti implants (topography, chemistry and wettability) influence biological responses at the interface between the bone tissue and the implant and, consequently, their osseointegration [4-9]. Therefore, any kind of methods to improve the osteoconductivity by modifying surface properties of Ti have been assessed. Some in vitro and in vivo studies have shown that modified surfaces achieved a higher early level of cell attachment than the untreated Ti surface.
Various surface modifications, including chemical treatment [10-12], thermal treatment [13], electrochemical method [14] and anodizing [15] and hydrothermal [16] have been tried to obtain a bioactive titanium oxide layer on the metal surface. Among above methods, anodizing and hydrothermal processes exhibit simple and effective surface treatment methods used to provide Ti implants with bioactive surface composition. It has been reported that by thermal oxidation or anodic oxidation, a thick Ti oxide layer produced diminished metal ion release, which can improve the in vitro biocompatibility and bone formation of Ti6Al4V alloy implants [17-20]. It has been shown that hydrothermal treatment of anodized Ti-substrate with calcium glycerophosphate and calcium acetate as electrolytes led to higher protein production than with untreated Ti surface [21].
Surface roughness and hydrophilicity are important factors of biomaterials which related with protein adsorption and cell adhesion after implantation into the body [22-26]. Yamamoto et al. reported that the anodizing process could produce a fine (Ra/µm < 0.1) and hydrophilic surface of Ti. Those properties could improve the osteoconductivity of TiO2 coating but, the additional surface treatment to produce a more hydrophilic surface was still needed because the water contact angle (WCA) of anodized TiO2 coatings did not lower than 20 (deg.) [27-30]. The hydrothermal process provided the surface of anodized TiO2 coating became more hydrophilic and the osteoconductivity increased about four times higher than untreated Ti [31].
However, the influence of surface hydrophilicity especially a super-hydrophilic surface on osteoconductivity by in vivo test has not been entirely clear yet. For that reason, in the present study, we analyzed whether the hydrothermal treatment using distilled water would create a hydrophilic surface on Ti samples to make better osteoconductivity. Moreover, we compared its osteoconductivity with as-polished and those of Ti implant produced by various surface modifications, such as anodizing and anodizing followed by hydrothermal. The osteoconductivity was evaluated in in vivo test.
2. Materials and Methods
2.1. Pre-Treatment of Ti Substrates
Commercially pure Ti (cp-Ti) plates with surface area = 1.1 cm2 and cp-Ti rods with 2 mm in diameter and 5 mm in length were used as substrates to evaluate surface properties and in vivo testing of as-polished, as-anodizing, as-hydrothermal and anodizing + hydrothermal. The substrates were ground with emery papers up to #2000, and followed by polishing using Al2O3 particles with 0.05 µm in size. Then, they were cleaned and degreased with ethanol in an ultrasonic cleaner, and finally dried in air. After this pre-treatment, following treatments were carried out.
2.2. Hydrothermal Process
The hydrothermal process was applied to cp-Ti after polishing. The samples were immersed in a beaker of 300 ml distilled water and put in an autoclaving unit. The temperature of hydrothermal vessel was set at 453 K and kept at this condition for 180 min. Regarding to a previous research, the hydrothermal treatment performed at a temperature of 453 K for 180 min was sufficient to produce a small and stable WCA [30]. After hydrothermal treatment, the beaker was directly taken out from autoclave unit and the samples were cooled naturally to the room temperature in the baker.
2.3. Anodizing Process
The Ti substrate after polishing was used for anodizing process. A Ti substrate and a Pt coil were used as the anode and cathode, respectively. The electrolyte for anodizing treatment was 0.1 M H2SO4 aqueous solution with pH around 1.0. The anodizing process was performed by applied voltage up to 100 V at 0.1 Vs−1 at room temperature as reported by Yamamoto et al. [27]. This processing could produce a TiO2 coating film with Ra (arithmetical means of roughness) < 0.1 µm.
2.4. Anodizing + Hydrothermal Process
The anodized Ti plate was hydrothermally treated at 453 K for 180 min in 300 ml distilled water using an autoclaving unit.
2.5. Sample Storage
After any treatments, the samples were sterilized at temperature 394 K for 20 min. Then, the surface treated samples were kept in the following three conditions at room temperature in air, in distilled water and in five times concentrated phosphate buffered saline (5PBS(−), pH 7.5). The composition of 5PBS(−) was 685 NaCl, 13.5 KCl, 50 Na2HPO4, and 8.8 KH2PO4 in mM.
2.6. Surface Characterization
The surface morphologies of the all samples after various processes were observed using scanning electron microscope (SEM). The coated films were determined by thin-film X-ray diffraction (XRD) and an X-ray photoelectron spectroscopy (XPS). The surface roughness was measured by means of contactless probing using a confocal laser scanning microscope with a measurement area of 150 µm × 112 µm and was expressed as the arithmetical means of the surface roughness (Ra). The WCA was estimated using a 2 µL droplet of distilled water
2.7. In Vivo Test
All rod samples after various processes and keeping in different storages were subjected to in vivo testing. Then, they were implanted in rats’ tibia for 14 d [32]. The substrates were slices toward longitude and stained with toluidine blue. By optical microscope, the interface between implant and the cortical bone was observed and also the cancellous bone. The sum of the linear bone contact with the implant surface was measured and was expressed as percentage over the entire implant length (the bone-implant contact ratio, RB-I) in the cancellous bone and the cortical bone parts. Significant differences in the bone-implant contact ratio were examined statistically using Tukey-Kramer method [33]. Differences were considered statistically significant at the p < 0.05 level.
3. Results and Discussion
3.1. Surface Characterization of Investigated Samples
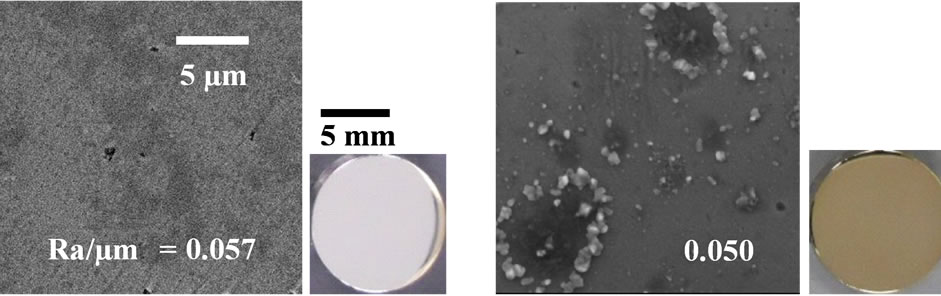
The SEM, optical micrograph and surface roughness (Ra) of samples are displayed in Figure 1. Compared to aspolished samples (a), the sample surfaces of as-anodizing (b), as-hydrothermal (c) and as-anodized + hydrothermal (d) show a change of color become yellow as an interference color, which indicates that an oxidation was occurred during process. This phenomenon was proved by XRD analysis where there was found only a peak of TiO2 (anatase) in all treated samples except as-polished sample as shown in Figure 2. However, the intensity of the anatase peak of hydrothermal samples (c) becomes weak and it cannot be detected more clearly compared with the other samples. It is probably because the oxide layer formed by only hydrothermal treatment was too thin. Besides, all the sample surfaces were non porous and only some fine particles attached to the hydrothermal surfaces. Although three types of treated samples (asanodized, anodized + hydrothermal and as-hydrothermal samples) contribute to slight increase in surface roughness compared to as-polished, the smooth surfaces still kept maintain with Ra/µm < 0.1. It can be said that the anodizing and hydrothermal processes give no significant effect on the surface roughness, so we can ignore Ra ef-
 (a)
(a)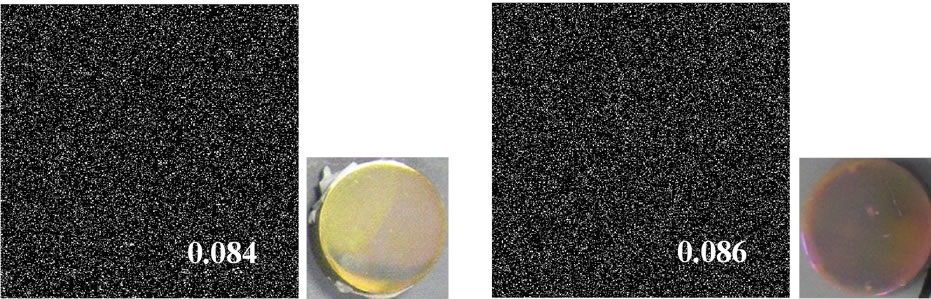 (b)
(b)
Figure 1. Surface morphology, optical micrograph and surface roughness (Ra) of Ti samples processed by different surface modification methods: (a) as-polished; (b) as-anodized; (c) as-hydrothermal and (d) anodized + hydrothermal.
fect on in vivo testing.
On the other hand, a contrast phenomenon is shown in wettability (Figure 3) where the WCA of the as-polished sample decreased from 71 (deg.) to 28 (deg.) after anodizing. Then, the sample surfaces of hydrothermal with and without anodizing become more hydrophilic when the WCA continued to decrease until 13 and 10 (deg.).
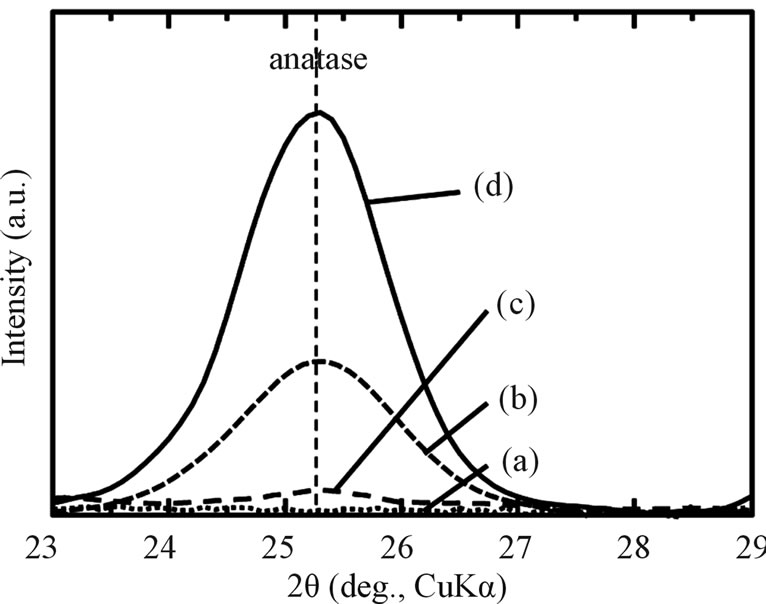
Figure 2. XRD patterns of Ti samples with various surface modifications: (a) as-polished; (b) as-anodized; (c) as-hydrothermal; and (d) anodized + hydrothermal.
 (A)
(A) (B)
(B)
Figure 3. The relationship between WCA and (A) the number of OH group and (B) the amount of adsorbed hydrocarbon on Ti samples with various surface modifications: (a) as-polished; (b) as-anodized; (c) as-hydrothermal; and (d) anodized + hydrothermal.
Regarding to previous research, the high WCA can be caused by adsorption of hydrocarbon which come from air atmosphere [34]. In a fact, determination of the influence of adsorbed oxygen (O) and carbon (C) on the treated samples were analyzed using XPS. From Figure 3, it shows that all of the investigated samples consisted primarily of titanium (Ti) and oxygen (O). Carbon (C) was detected as surface contaminant in the XPS analysis. The O 1s XPS spectrum deconvoluted into three peaks (530.1, 531.5, and 532.5 eV) originated from anhydrous oxide (O2−), hydroxyl group (OH−) and hydrate and/or adsorbed water (H2O), in the same way as in a previous report [35]. Meanwhile, the C 1s spectrum also contained three peaks originating from C-H, C-O, and C = O. The ratios of the proportion of OH− to that of TiO2, [OH−]/ [TiO2] and C-H to that of TiO2, [C-H]/[TiO2] of the surface modified titanium in different processes are shown in Figure 3. The [OH−]/[TiO2] values in the surface layers modified with anodizing process in H2SO4 solution are smaller than those without the treatment (as-polished). Then, this value decreased slightly after additional hydrothermal treatment. These results show that the amount of OH group on anodized sample was not change and affected by hydrothermal process. In contrast, the [C-H]/ [TiO2] values of as-polished and as-anodized samples are almost similar and then continue to reduce after additional hydrothermal treatment on anodized titanium samples. This trend also can be found in titanium samples after applying only hydrothermal process without anodizing. It suggested that the reason why the titanium surface becomes more hydrophilic when hydrothermal process was applied to the sample was because of the reduction in adsorbed hydrocarbon.
Storing samples in different environments will influence their WCA. It can be seen in Figure 4, when the all treated samples stored in the air (A), the WCA increased by increasing of the storage time. This fact also is shown to the samples stored in the distilled water (B). However, this tendency became reverse for all investigated samples when they stored in the PBS(−) especially in a higher concentration of PBS(−) solution, 5PBS(−) (C). Their WCA became decreased by increasing of the storage time. The difference of WCA with time in the various storage environments were effected by chemical species on the surface [31]. From the same figure, we also can see that the WCA of hydrothermal sample is lower than that of as-anodized and anodized + hydrothermal samples after keeping in the air, distilled water and 5PBS(−). The contamination was removed by hydrothermal treatment at high temperature, high pressure and became a clean surface, so that, the hydrophilicity was improved likely. A drastically lessening of WCA of as-hydrothermal samples to become less than 10 (deg.) was occurred when the samples immersed into the 5PBS(−). It can be indicated
 (A)
(A)  (B)
(B)  (C)
(C)
Figure 4. Changes in WCA under different storing conditions and periods: (A) in air; (B) in distilled water; and (C) in 5PBS (−) on Ti samples processed with various surface modifications (○: as-anodized; ●: anodized + hydrothermal; and △: as-hydrothermal.
that by immersing each substrate in 5PBS(−), the sample surface became super-hydrophilic. Various inorganic solute ions such as Na+ and Cl− in high concentration 5PBS(−) were absorbed on the hydrothermaled clean surface, and has improved more hydrophilic, as likely [31].
3.2. In Vivo Analysis
Yamamoto et al. explained that the bone-implant contact ratio, RB-I of the anodized specimens in various aqueous solutions for both the cortical bone part and thecan-cellous bone part had same trend, but the metabolism in cancellous bone was faster than the cortical bone and it took a long time to see the bone reaction to anodized specimens (14 d) [30]. Therefore, in this analysis, we focus on the RB-I values from the cortical bone part.
Titanium substrates after various processes and different storage condition were subjected to in vivo testing and their osteoconductivity were observed. Figure 5 shows the effect of the WCA on the osteoconductivity (RB-I in the cortical bone) of pure titanium in different processes. As a comparison, we used a previous in vivo data for TiO2-coated samples after anodizing in several electrolyte solutions with Ra/µm < 0.1 [27,29,30]. This figure explains that as-polished sample which hydrophobic surface has low osteoconductivity (12%). By decreasing of WCA, the osteoconductivity of as-polished titanium was increased after applying anodizing (43%) and additional hydrothermal process on anodized sample (49%). The notable improvement of osteoconductivity was detected when the hydrothermal sample immersed into the 5PBS(−) and become a super-hydrophilic surface. Even though there was a small difference of the RB-I between as-anodized + hydrothermal (58%) and as-hydrothermal samples (50%) after immersing them into 5PBS (−), it can be said that a better osteoconductivity can be achieved by applying hydrothermal process even though without anodizing.
Factors which have essential influence in the osteoconductivity are surface roughness, contact angles or wettability and surface energy. However, surface roughness was nearly constant in this experiment. A high contact angle with a hydrophobic surface generates poor cell attachment and as a contrary, a low contact angle contributes to high surface energy, which is another factor that provides to better cell attachment. Hydrophilicity is a crucial property of implants which enhances the adhe-

Figure 5. Relationship between RB-I value (in cortical bone) and WCA of Ti samples with various surface modifications and subsequent storage methods: ■ as-polished; ○ anodized in different aqueous solutions (Ra/μm < 0.1); ● anodized + hydrothermal, then stored in distilled water; ◆ anodized + hydrothermal, then stored in 5PBS(−) solution; and △ ashydrothermal, then stored in 5PBS(−) solution.
sion, spreading, and proliferation of cells on their surfaces. Surface hydrophilicity also influences the adsorption of cell adhesion proteins containing Arg-Gly-Asp (RGD), such as fibronectin, on the surface of implants, and as a consequence strengthens the adhesion and spreading of osteoblast precursors on implant surfaces [22,36]. By increasing the adsorption of these RGD-containing extracellular matrix proteins and improving subsequent cell behavior on these surfaces, highly wettable surfaces can improve the early bone healing process at the cellbiomaterial interface [23,37,38]. Furthermore, hydrophilic surfaces can encourage the biomineralization process. In this study, hydrothermal process without anodizing which applied in to titanium substrates contributed the hydrophilic surface with lower WCA. It showed the enhanced of osteoconductivity compared to as-polished titanium. Furthermore, by immersing into 5 PBS(−), the hydrothermal sample surface become super-hydrophilic and it indicated a high osteoconductivity .
4. Conclusion
Anodizing and hydrothermal processes produced titanium surfaces with different surface characteristic. It showed that anodizing process cannot give critical influence to improve the hidrophilicity of pure titanium because although there was reduction of water contact angle after anodizing compared with as-polished sample, it still produced hydrophobic surface (WCA > 20 deg.). However, by applying hydrothermal process with or without anodizing, the sample surface becomes more hydrophilic (WCA < 20 deg.). Furthermore, although detailed studies are needed to elucidate the exact mechanism of the enhanced osteoconductivity, it was observed that the only hydrothermal process without anodizing which followed by immersion into 5PBS(−) produced a super-hydrophilic surface (WCA < 10 deg.) and it has higher osteoconductivity than anodized TiO2 in H2SO4 in part of cortical bone and it is an effective method in producing a high osteoconductive implant surface in the pure titanium.
5. Acknowledgements
This work was partially supported by Indonesian Government scholarship from Directorate General of Higher Education (DIKTI), and Project of Advanced Materials Development and Integration of Novel Structured Metallic and Inorganic Materials by MEXT.