Silica-Grafted Ionic Liquids as Recyclable Catalysts for the Synthesis of 3,4-Dihydropyrano[c]chromenes and Pyra-no[2,3-c]pyrazoles ()
1. Introduction
In the recent years, ionic liquids were used as solvents due to their particular properties, such as the ability to dissolve many organic and inorganic substances and undetectable vapor pressure [1]. In addition, Brønsted acidic task-specific ionic liquids (BAILs), such as those possessing  as a counter anion find a broad application in organic synthesis, acting as both solvents and catalysts. Keim and co-workers reported the synthesis of 1-butyl-3-methylimidazolium hydrogen sulfate ([bmim] HSO4) [2]. In addition, in the year of 2002 ([bmim]HSO4) was used as a catalyst in the Friedel-Crafts alkylation [3]. The other applications of these acidic ionic liquids such as acetalization and thioacetalization of carbonyl compounds [4], Fischer indole synthesis [5], acetylation of alcohols and phenols [6], preparation of azides from alcohols [7], selective nitration of phenols [8], synthesis of 1,8-dioxo-octahydroxanthenes [9], formylation of alcohols [10], synthesis of polysubstituted quinolines [11], have been proceeded with very good yields and selectivities. Recently, immobilization of acidic ionic liquids on solid supports has been designed and it can offer important advantages in handling, separation and reuse procedures. Based on economic criteria, it is desirable to minimize the amount of ionic liquid utilized in a potential process. Immobilized acidic ionic liquids have been used as novel solid catalysts, e.g., for esterification, nitration reactions [12], acetal formation [13], Baeyer-Villiger reaction [14], synthesis of α-aminonitriles [15] and bispyrazolones [16].
as a counter anion find a broad application in organic synthesis, acting as both solvents and catalysts. Keim and co-workers reported the synthesis of 1-butyl-3-methylimidazolium hydrogen sulfate ([bmim] HSO4) [2]. In addition, in the year of 2002 ([bmim]HSO4) was used as a catalyst in the Friedel-Crafts alkylation [3]. The other applications of these acidic ionic liquids such as acetalization and thioacetalization of carbonyl compounds [4], Fischer indole synthesis [5], acetylation of alcohols and phenols [6], preparation of azides from alcohols [7], selective nitration of phenols [8], synthesis of 1,8-dioxo-octahydroxanthenes [9], formylation of alcohols [10], synthesis of polysubstituted quinolines [11], have been proceeded with very good yields and selectivities. Recently, immobilization of acidic ionic liquids on solid supports has been designed and it can offer important advantages in handling, separation and reuse procedures. Based on economic criteria, it is desirable to minimize the amount of ionic liquid utilized in a potential process. Immobilized acidic ionic liquids have been used as novel solid catalysts, e.g., for esterification, nitration reactions [12], acetal formation [13], Baeyer-Villiger reaction [14], synthesis of α-aminonitriles [15] and bispyrazolones [16].
Dihydropyrano[c]chromenes and their derivatives are of considerable interest as they possess a wide range of biological properties [17], such as spasmolytic, diuretic, anti-coagulant, anti-cancer, and anti-anaphylactic activity [18,19]. In addition, they can be used as cognitive enhancers, for the treatment of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, AIDS associated dementia and Down’s syndrome as well as for the treatment of schizophrenia and myoclonus [20]. Also, a number of 2-amino-4H-pyranes are useful as photoactive materials [21]. A number of methods have been reported for the synthesis of 3,4-dihydropyrano[c] chromenes such as piperidine in organic solvent, i.e. ethanol and pyridine [22], K2CO3 under microwave irradiation [23], diammonium hydrogen phosphate [24], tetrabutylammonium bromide [25], sodium dodecyl sulfate [26], DBU [27], morpholine [28], α-Fe2O3 nanopatricles [29], CuO nanopatricles [30], and silica-bonded N-propylpiperazine sodium propionate [31].
In continuation of our studies on the design and application of acidic ionic liquids or silica-grafted ionic liquids as catalyst in organic transformations (Scheme 1) [8-11,15,16], herein, we describe the application of silicagrafted N-propyl-imidazolium hydrogen sulfate ([Sipim] HSO4) in the synthesis of pyrano[c]chromenes and pyrano[3,4c]pyrazoles.
2. Results and Discussion
2.1. Synthesis of Dihydropyrano[c]chromenes
To study the effect of catalyst loading on the synthesis of 2-amino-4-aryl-5-oxo-4H,5H-pyrano[3,2-c]chromene-3- carbonitrile the condensation reaction of malononitrile, 4-chlorobenzaldehyde, and 4-hydroxycoumarin was chosen as a model reaction (Table 1). The results show clearly that ionic liquids and silica-grafted ionic liquids (SGILs) are effective catalysts for this condensation and the optimal amount of SGILs was 0.1 g per 1 mmol of aldehyde under solvent-free conditions at 100˚C. The best result was obtained in the presence of [Sipim]HSO4. This condensation was carried out with the lower amounts of [Sipim]HSO4 0.05 g and 0.07 g and the corresponding product was obtained in 88% and 91% yield (Table 1, entries 4 and 5).
Also, this three-component condensation was accomplished in the presence of [Sipim]HSO4 in ethanol and water at reflux conditions in longer reaction time and lower yield (Table 1, entries 1 and 2). Moreover, the model reaction was examined under solvent-free conditions at 80˚C gave 1b after 90 min in 80% yield (Table 1, entry 8). The model reaction was reacted in the presence of N-(3-silicapropyl) imidazolium chloride ([Sipim]Cl), N-(3-silicapropyl) imidazolium dihydrogen phosphate ([Sipim]H2PO4), and N-(3-silicapropyl) imidazolium triflate ([Sipim]OTf), under solvent-free conditions at 100˚C in 80%, 80%, and 75% yield respectively (Table 1, entries 10-12). In addition, the same model reaction was carried out in the presence of ionic liquids such as imidazolium chloride, imidazolium hydrogen sulfate, methylimidazolium hydrogen sulfate, and imidazolium bromide under solvent-free and at 100˚C in 85%, 80%, 80%, and 85% yield respectively (Table 1, entries 13-17).

Scheme 1. Preparation of silica-grafted propyl imidazolium hydrogen sulfate ([Sipim]HSO4).

Table 1. Investigation the effect of catalyst and solvent on the reaction of 4-chlorobenzaldehyde, malononitrile and 4- hydroxycoumarin.
The synthesis of 2-amino-4-aryl-5-oxo-4H,5H-pyrano- [3,2-c]chromene-3-carbonitrile was achieved by the threecomponent condensation of an aromatic aldehyde, malononitrile, and 4-hydroxycoumarin in the presence of [Sipim]HSO4 (0.1 g, 0.08 mmol of H+ [15]) under solventfree conditions at 100˚C (Table 2).
Thereafter, a series of different 3,4-dihydropyrano- [c]chromene derivatives were prepared successfully from different aromatic aldehydes bearing electron-withdrawing and electron donating groups, 4-hydroxycoumarin and malononitrile under solvent-free conditions. Electron-withdrawing groups such as 3-nitro, 4-nitro, and 2-nitro-benzaldehyde reacted under optimized conditions into corresponding 1f, 1g, and 1h in 93%, 90%, and 89% yield after 30 min (Table 2, entries 6-8). Electrondo-
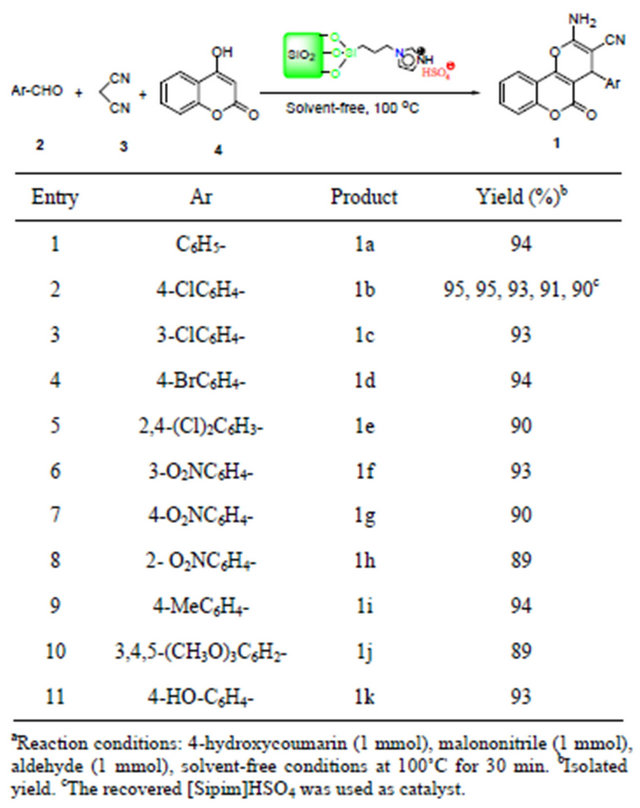
Table 2. [Sipim]HSO4 catalyzed synthesis of dihydropyrano [c]chromene derivatives.a
nating groups such as 4-Me, 3,4,5-(MeO)3-benzaldehyde were treated with malononitrile and 4-hydroxycoumarin under optimized conditions gave corresponding products 1i and 1j in high yields (Table 2, entries 9 and 10). The results clearly indicate that reactions can tolerate a wide range of differently substituted aromatic aldehydes.
The possibility of recycling the catalyst was examined using the reaction of malononitrle, 4-chlorobenzaldehyde and 4-hydroxycoumarin under the optimized conditions. Upon completion, the reaction mixture was washed with warm ethanol (3 × 30 mL). The recovered catalyst was dried and reused for subsequent runs. The recycled catalyst could be reused fourth times without any additional treatment. No observation of any appreciable loss in the catalytic activity of [Sipim]HSO4 was made (Table 2, entry 2).
2.2. Synthesis of Pyrano[2,3-c]pyrazoles
Condensed pyrazoles are also biologically interesting compounds and their chemistry has recently received considerable attention [32,33]. Several pyrano[2,3-c] pyrazoles are reported to have useful biological effects, such as analgesic and anti-inflammatory activities [34]. Moreover, the biological activity of fused azoles has led to intensive research on their synthesis [35,36]. Recently, three component one-pot condensation of 3-methyl-1- phenyl-1H-pyrazol-5(4H)-one, aldehydes and malononitrile for the construction of 1,4-dihydropy-rano[2,3-c] pyrazole derivatives has been reported under different conditions [37-41]. However, most of the reported methods have their drawbacks. For example, hexadecyltrimethylammonium bromide (HTMAB) is a harmful and irritant catalyst which is dangerous for the environment [39]. In addition, the reusability of the catalysts such as D,L-proline is not reported in a number of cases [40]. Heteropoly acids, silica-supported bases or acids have been reported the synthesis of 1,4-dihydropyrano[2,3-c] pyrazole derivatives via multi-component condensation reaction [42-48].
The scope of this methodology was extended to the synthesis of pyrano[2,3-c]pyrazols. Initially three-component condensation reaction of malononitrile, 4-chlorobenzaldehyde, and 3-methyl-1-phenyl-1H-pyrazol-5 (4H)- one was chosen as a model reaction and the effect of different catalysts was investigated at 110˚C under solvent-free conditions (Table 3).
Again, the results show clearly that ionic liquids and silica-grafted ionic liquids (SGILs) are effective catalysts
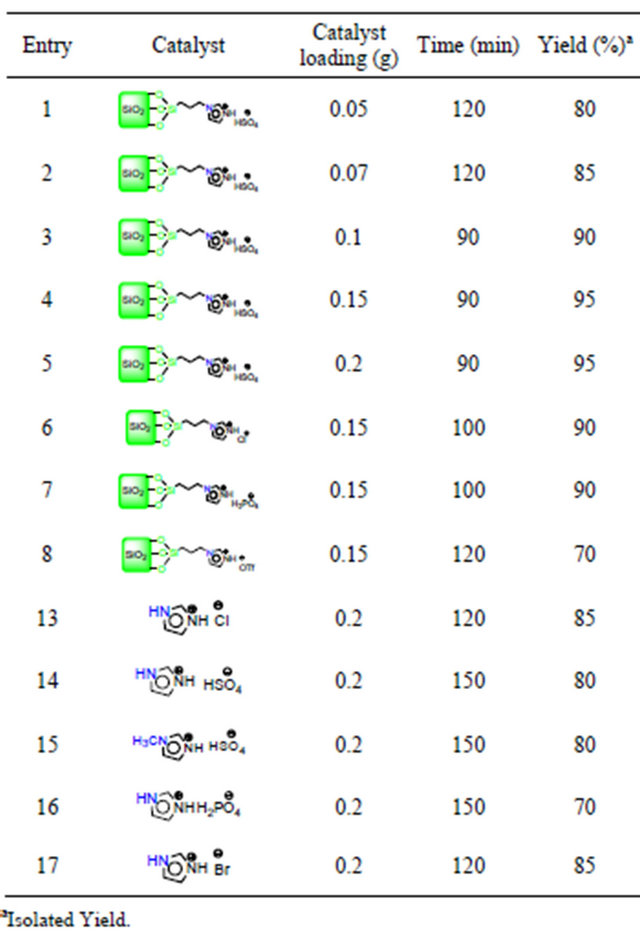
Table 3. Investigation the effect of catalyst on the reaction of 4-chlorobenzaldehyde, malononitrile and 3-methyl-1- phenyl-1H-pyrazol-5(4H)-one at 110˚C under solvent-free conditions.
for this condensation and the optimal amount of SGILs was 0.15 g per 1 mmol of aldehyde under solvent-free conditions at 110˚C.
A range of different substituted groups on aromatic aldehydes involving electron-withdrawing groups such as 3-nitro, 4-nitro, and electron-donating groups such as 3,4- dimethoxy-benzaldehyde reacted under optimized conditions into corresponding 5f, 5g, and 5h in 90%, 88%, and 90% yield after 90 min (Table 4, entries 6-8). 4-Hydroxy-benzaldehyde was treated with malononitrile and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one under optimized conditions gave corresponding products 5i in 90% yield (Table 4, entry 9).
The possibility of recycling the catalyst was examined using the reaction of malononitrle, 4-chlorobenzaldehyde and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one under the optimized conditions. Upon completion, the reaction mixture was washed with warm ethanol (3 × 30 mL). The recovered catalyst was dried and reused for subsequent runs. The recycled catalyst could be reused fourth times without any additional treatment. No observation of any appreciable loss in the catalytic activity of [Sipim]HSO4 was made (Table 4, entry 2).
3. Experimental Section
3.1. General
Chemicals were purchased from Fluka, Merck and Aldrich Chemical Companies. All the products were char-
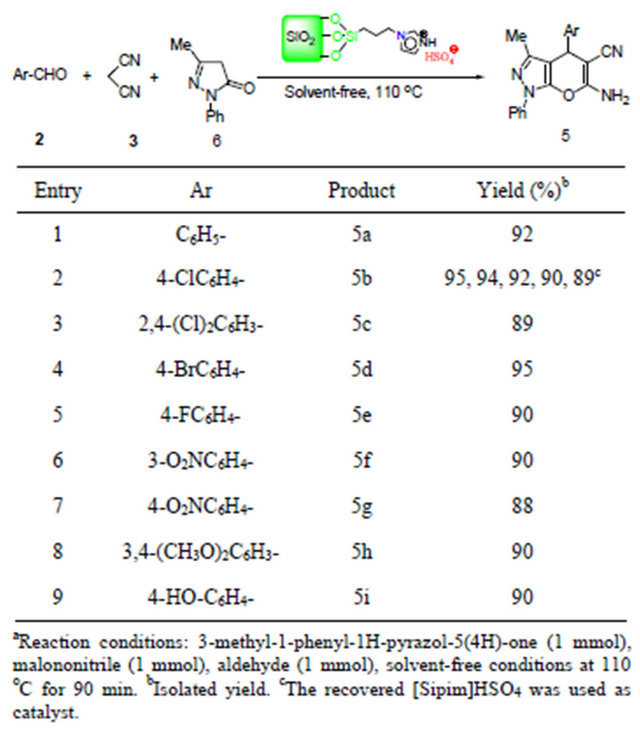
Table 4. [Sipim]HSO4 catalyzed synthesis of dihydropyrano [2,3-c]pyrazole derivatives.a
pyl-imidazolium hydrogen sulfate ([Sipim]HSO4) was acterized by comparison of their IR, 1H NMR and 13C NMR spectroscopic data and their melting points with the reported values [24-31,38-41]. Silica-grafted N-proprepared according to our previous reported procedure [15].
3.2. General Procedure for the Synthesis of 3,4-Dihydropyrano[c]chromenes
To a mixture of aromatic aldehyde (1 mmol), malonitrile (1 mmol), and 4-hydroxycoumarin (1 mmol), catalyst [Sipim]HSO4 (0.1 g, 0.08 mmol of H+) was added and the mixture was heated at 100˚C under solvent-free conditions. After completion of the reaction, as indicated by TLC, ethanol (10 mL) was added and the reaction mixture was filtered. The remaining was washed with warm ethanol (3 × 5 mL) in order to separate heterogeneous catalyst. After cooling the crude products were precipitated. The crude products were purified by recrystallization from ethanol (95%). The recovered catalyst was dried and reused for subsequent runs.
2-Amino-4-phenyl-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile 1a: mp 258˚C - 260˚C, (Lit.: 256˚C - 258˚C, [24]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.46 (s, 1 H), 7.23 - 7.27 (m, 3 H), 7.31 - 7.35 (m, 2 H), 7.43 - 7.52 (m, 2H), 7.72 (dt, 1 H, J1 = 7.8 Hz, J2 = 1.6 Hz), 7.92 (dd, 1 H, J1 = 8.0 Hz, J2 = 0.8 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 58.41, 104.47, 113.42, 117.06, 119.68, 122.98, 125.18, 127.62, 128.10, 129.01, 133.45, 143.80, 152.60, 153.89, 158.36, 158.40, 160.05.
2-Amino-4-(4-chlorophenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile 1b: mp 263˚C - 265˚C, (Lit.: 252˚C - 255˚C, [28]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.49 (s, 1 H), 7.31 (d, 2 H, J = 8.4 Hz), 7.38 (d, 2 H, J = 8.4 Hz), 7.45 - 7.51 (m, 2 H), 7.72 (t, 1 H, J = 7.8 Hz), 7.91 (d, 1 H, J = 8.0 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 57.94, 103.94, 113.39, 117.06, 119.54, 123.01, 125.18, 128.92, 130.12, 132.18, 133.51, 142.80, 152.64, 154.02, 158.34, 158.38, 160.03.
2-Amino-4-(3-chlorophenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile 1c: mp 241˚C - 243˚C, (Lit.: 246˚C - 248˚C, [26]). 1H NMR (400 MHz, DMSOd6): δ (ppm) 4.52 (s, 1 H), 7.26 (d, 1 H, J = 7.2 Hz), 7.31 - 7.38 (m, 3 H), 7.47 - 7.53 (m, 2 H), 7.73 (t, 1 H, J = 7.6 Hz), 7.91 (d, 1 H, J = 7.6 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 57.81, 103.63, 113.43, 117.02, 119.55, 123.06, 125.13, 127.03, 127.66, 128.06, 130.87, 133.48, 133.54, 146.26, 152.66, 154.19, 158.37, 158.42, 160.06.
2-Amino-4-(4-bromophenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile (1d): mp 254˚C - 256˚C, (Lit.: 247˚C - 249˚C, [26]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.48 (s, 1 H), 7.25 (d, 2 H, J = 8.0 Hz), 7.46 - 7.52 (m, 4 H), 7.73 (t, 1 H, J = 7.4 Hz), 7.91 (d, 1 H, J = 7.6 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 57.85, 103.88, 113.39, 117.08, 119.54, 120.71, 123.02, 125.20, 130.50, 131.85, 133.54, 143.23, 152.65, 154.03, 158.32, 160.04.
2-Amino-4-(2,4-dichloro-phenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile (1e): mp 258˚C - 259˚C, (Lit.: 253˚C - 255˚C, [30]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.98 (s, 1 H), 7.35 - 7.41 (m, 2 H), 7.48 - 7.54 (m, 4 H), 7.60 (d, 1 H, J = 2.0 Hz), 7.74 (dt, 1H, J1 = 8.0 Hz, J2 = 1.6 Hz), 7.92 (dd, 1 H, J1 = 8.0 Hz, J2 = 1.6 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 34.33, 56.43, 102.96, 113.29, 117.12, 119.18, 123.04, 125.25, 128.36, 129.34, 132.56, 132.88, 133.61, 133.82, 139.91, 152.70, 154.62, 158.57, 159.94.
2-Amino-4-(3-nitrophenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile (1f): mp 266˚C - 267˚C, (Lit.: 262˚C - 264˚C, [24]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.74 (s, 1 H), 7.46 - 7.53 (m, 2 H), 7.58 (s, 2H, NH2), 7.64 (t, 1H, J = 8.0 Hz), 7.74 (dt, 1 H, J1 = 7.9 Hz, J2 = 1.4 Hz), 7.82 (d, 1 H, J = 7.6 Hz), 7.93 (dd, 1 H, J1 = 8.0 Hz, J2 = 1.2 Hz), 8.12 - 8.15 (m, 2 H). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 57.36, 103.33, 113.39, 117.07, 119.45, 122.77, 122.93, 123.10, 125.19, 130.56, 133.61, 135.26, 145.96, 148.30, 152.73, 154.35, 158.52, 158.57, 160.10.
2-Amino-4-(4-nitrophenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile (1g): mp 259˚C - 261˚C, (Lit.: 258˚C - 260˚C, [24]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.68 (s, 1 H), 7.47 - 7.54 (m, 2 H), 7.58 - 7.61 (m, 2 H), 7.75 (dt, 1 H, J1 = 8.0 Hz, J2 = 1.2 Hz), 7.93 (dd, 1H, J1 = 8.0 Hz, J2 = 1.2 Hz), 8.21 (d, 2 H, J = 8.4 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 57.20, 103.27, 113.36, 117.13, 119.34, 123.09, 124.22, 125.26, 129.67, 133.69, 147.08, 151.23, 152.74, 154.42, 158.43, 158.47, 160.07.
2-Amino-4-(2-nitrophenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile (1h): mp 258˚C - 260˚C, (Lit.: 258˚C - 260˚C, [31]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 5.25 (s, 1 H), 7.46 - 7.59 (m, 6 H), 7.67 (t, 1 H, J = 7.6 Hz), 7.73 (t, 1 H, J = 7.8 Hz), 7.91 (d, 2 H, J = 7.6 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 56.52, 103.75, 113.27, 117.09, 119.22, 123.03, 124.45, 125.21, 128.93, 131.65, 133.57, 134.16, 137.83, 149.66, 152.63, 154.06, 159.08, 160.19.
2-Amino-4-p-tolyl-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile (1i): mp 253˚C - 255˚C, (Lit.: 259˚C - 260˚C, [28]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 2.27 (s, 3H), 4.41 (s, 1 H), 7.11 - 7.15 (m, 4 H), 7.40 (s, 2 H, NH2), 7.46-7.52 (m, 2 H), 7.72 (dt, 1 H, J1 = 7.8 Hz, J2 = 1.6 Hz), 7.91 (dd, 1 H, J1 = 7.8 Hz, J2 = 1.4 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 21.10, 58.53, 104.59, 113.42, 117.03, 119.71, 122.94, 125.15, 128.00, 129.55, 133.38, 136.78, 140.86, 152.56, 153.72, 158.30, 160.01.
2-Amino-4-(3,4,5-trimethoxy-phenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile (1j): mp 236˚C - 238˚C, (Lit.: 236˚C - 238˚C, [31]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 3.64 (s, 3 H), 3.72 (s, 6 H), 4.44 (s, 1 H), 6.53 (s, 2 H), 7.41 (s, 2 H, NH2), 7.47-7.52 (m, 2 H), 7.73 (dt, 1H, J1 = 8.0 Hz, J2 = 1.6 Hz), 7.91 (dd, 1 H, J1 = 8.0 Hz, J2 = 1.6 Hz). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 56.36, 58.32, 60.39, 104.11, 105.38, 113.54, 117.05, 119.71, 123.04, 125.11, 133.39, 137.03, 139.46, 152.64, 153.30, 153.98, 158.38, 160.14.
2-Amino-4-(4-hydroxy-phenyl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile (1k): mp 266˚C - 268˚C, (Lit.: 260˚C - 263˚C, [28]). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.33 (s, 1 H), 6.71 (d, 2 H, J = 8.4 Hz), 7.06 (d, 0.72 H, J = 8.8 Hz), 7.36 (s, 2 H, NH2), 7.43 - 7.49 (m, 2 H), 7.69 (dt, 1H, J1 = 7.8 Hz, J2 = 1.6 Hz), 7.89 (dd, 1 H, J1 = 7.8 Hz, J2 = 1.4 Hz), 9.41 (s, 1H, OH). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 58.84, 104.92, 113.43, 115.59, 116.96, 119.87, 122.89, 125.09, 129.20, 133.25, 134.23, 152.48, 153.42, 156.80, 158.32, 160.02.
3.3. General Procedure for the Synthesis of Dihydropyrano[2,3-c]pyrazoles
To a mixture of aromatic aldehyde (1 mmol), malonitrile (1 mmol), and 3-methyl-1-phenyl-1H-pyrazol-5(4 H)-one (1 mmol), catalyst [Sipim]HSO4 (0.15 g, 0.12 mmol of H+) was added and the mixture was heated at 110˚C under solvent-free conditions. After completion of the reaction, as indicated by TLC, ethanol (10 mL) was added and the reaction mixture was filtered. The remaining was washed with warm ethanol (3 × 5 mL) in order to separate heterogeneous catalyst. After cooling the crude products were precipitated. The crude products were purified by recrystallization from ethanol (95%). The recovered catalyst was dried and reused for subsequent runs.
6-Amino-3-methyl-1,4-diphenyl-1,4-dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5a): mp 170˚C - 171˚C, (Lit.: 168˚C - 170˚C, [38]). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.92 (s, 3 H), 4.69 (s, 1 H), 4.71 (s, 2 H, NH2), 7.27 - 7.40 (m, 6 H), 7.49 (t, 2 H, J = 7.8 Hz), 7.68 (d, 2H, J = 8.0 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 12.94, 37.44, 63.88, 98.36, 119.14, 121.22, 126.80, 127.62, 127.91, 128.83, 129.31, 137.57, 142.00, 143.84, 146.46, 158.17.
6-Amino-4-(4-chlorophenyl)-3-methyl-1-phenyl-1,4- dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5b): mp 174˚C - 176˚C, (Lit.: 174˚C - 177˚C, [43]). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.92 (s, 3 H), 4.68 (s, 1 H), 4.74 (s, 2 H, NH2), 7.22 (d, 2H, J = 8.4 Hz), 7.33 - 7.37 (m, 3 H), 7.49 (t, 2 H, J = 7.8 Hz), 7.67 (d, 2 H, J = 8.0 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 12.97, 36.91, 63.36, 97.87, 118.95, 121.25, 126.92, 129.06, 129.28, 129.34, 133.44, 137.45, 140.57, 143.80, 146.28, 158.23.
6-Amino-4-(2,4-dichlorophenyl)-3-methyl-1-phenyl- 1,4-dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5c): mp 183˚C - 185˚C, (Lit.: 182˚C - 184˚C, [38]). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.92 (s, 3 H), 4.78 (s, 2 H, NH2), 5.29 (s, 1H), 7.17 (d, 1 H, J = 8.4 Hz), 7.26 - 7.28 (m, 1 H), 7.35 (t, 1 H, J = 7.2 Hz), 7.46 - 7.51 (m, 3 H), 7.67 (d, 2 H, J = 8.0 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 12.77, 33.53, 61.98, 97.58, 118.62, 121.28, 126.97, 128.04, 129.35, 129.68, 131.49, 133.95, 137.41, 137.86, 143.95, 146.04, 158.84.
6-Amino-4-(4-bromophenyl)-3-methyl-1-phenyl-1,4- dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5d): mp 182˚C - 184˚C, (Lit.: 176˚C - 177˚C, [40]). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.92 (s, 3H), 4.67 (s, 1 H), 4.73 (s, 2H, NH2), 7.17 (d, 2H, J = 8.0 Hz), 7.35 (t, 1 H, J = 7.4 Hz), 7.48 - 7.53 (m, 4H), 7.67 (d, 2 H, J = 7.6 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 12.98, 36.98, 63.31, 97.78, 118.92, 121.26, 121.60, 126.93, 129.34129.63, 132.01, 137.45, 141.08, 146.28, 158.22.
6-Amino-4-(4-fluorophenyl)-3-methyl-1-phenyl-1,4- dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5e): mp 170˚C - 171˚C, (Lit.: 167˚C - 168˚C, [38]). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.91 (s, 3H), 4.69 (s, 1 H), 4.73 (s, 2 H, NH2), 7.07 (t, 2 H, J = 8.6 Hz), 7.24 - 7.28 (m, 2 H), 7.35 (t, 1 H, J = 7.4 Hz), 7.49 (t, 2H, J = 7.8 Hz), 7.67 (d, 2H, J = 8.0 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 12.94, 36.78, 63.69, 98.15, 115.86, 119.01, 121.24, 126.88, 129.33, 129.54, 137.49, 143.77, 146.32, 158.14, 162.17 (d, JC-F = 244.0 Hz).
6-Amino-3-methyl-4-(3-nitrophenyl)-1-phenyl-1,4-dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5f): mp 190˚C - 191˚C, (Lit.: 190˚C - 192˚C, [43]). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.92 (s, 3 H), 4.84 (s, 3H, CH & NH2), 7.37 (t, 1 H, J = 7.4 Hz), 7.51 (t, 2 H, J = 7.6 Hz), 7.60 (t, 1 H, J = 8.0 Hz), 7.67 - 7.70 (m, 3 H), 8.14 (s, 1 H), 8.21 (d, 1 H, J = 8.0 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 13.01, 37.38, 62.11, 97.21, 118.76, 121.42, 122.87, 127.11, 129.37, 129.89, 134.17, 137.33, 144.49, 145.92, 148.76, 158.75.
6-Amino-3-methyl-4-(4-nitrophenyl)-1-phenyl-1,4-dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5g): mp 194˚C - 196˚C, (Lit.: 195˚C - 197˚C, [43]). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.92 (s, 3 H), 4.83 (s, 3 H, CH & NH2), 7.37 (t, 1 H, J = 7.4 Hz), 7.47 - 7.53 (m, 4 H), 7.78 (d, 2 H, J = 8.0 Hz), 8.27 (d, 2 H, J = 8.4 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 12.98, 37.33, 62.26, 97.07, 118.58, 121.35, 124.28, 127.16, 128.88, 129.40, 137.30, 143.83, 146.00, 147.46, 149.19, 158.56.
6-Amino-4-(3,4-dimethoxyphenyl)-3-methyl-1-phenyl-1,4-dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5h): mp 167˚C - 169˚C, (Lit.: 193˚C - 195˚C, [38]). 1H NMR (400 MHz, CDCl3): δ (ppm) 1.91 (s, 3 H), 3.85 (s, 3 H), 3.87 (s, 3 H), 4.62 (s, 1 H), 4.73 (s, 2 H, NH2), 6,73 (d, 1H, J = 1.6 Hz), 6.79 - 6.85 (m, 2H), 7.31 (t, 1 H, J = 8.0 Hz), 7.45 (t, 2H, J = 8.0 Hz), 7.66 (d, 2 H, J = 8.4 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) 12.98, 37.06, 55.86, 55.98, 63.92, 98.29, 110.82, 111.12, 119.22, 120.18, 121.05, 126.71, 129.28, 134.56, 137.57, 143.80, 146.52, 148.41, 149.20, 158.02.
6-Amino-4-(4-hydroxyphenyl)-3-methyl-1-phenyl-1,4-dihydro-pyrano[2,3-c]pyrazol-5-carbonitrile (5i): mp 206˚C - 208˚C, (Lit.: 206˚C - 207˚C, [40]). 1H NMR (500 MHz, DMSO-d6): δ (ppm) 1.78 (s, 3 H), 4.55 (s, 1 H), 6.71 (d, 2 H, J = 7.8 Hz), 7.03 (d, 2 H, J = 7.8 Hz), 7.10 (s, 2 H, NH2), 7.31 (t, 1 H, J = 7.0 Hz), 7.48 (t, 2 H, J = 7.3 Hz), 7.77 (d, 2 H, J = 7.7 Hz), 9.31 (s, 1 H). 13C NMR (125 MHz, DMSO-d6): δ (ppm) 13.45, 36.88, 59.68, 99.91, 116.07, 120.75, 120.96, 126.94, 129.62, 130.18, 134.79, 138.45, 144.65, 146.22, 157.15, 160.305.
4. Acknowledgements
We are thankful to Persian Gulf University Research Council for partial support of this work.
NOTES