Effect of Antimony Additions on Corrosion and Mechanical Properties of Sn-Bi Eutectic Lead-Free Solder Alloy ()
1. Introduction
Due to the toxicity and harmful environmental effect of Pb present in tin solders, legislation trend to reduce or eliminate the utilization of lead from a wide variety of uses. There are many different metals and metal alloys that can be used as solders and a set of binary alloys have been chosen as candidates for lead-free solders: Sn-Bi, Sn-Ag, Sn-Zn, Sn-Cu and Sn-Sb. Among the ternary compositions, the Sn-Bi-Zn one was used in making printing wiring boards [1], however all of these eutectic compositions have a melting temperature above 200˚C. Sn-Zn eutectic has a lower melting temperature of 199˚C, but its corrosion behavior and poor wetting ability renders it useless [2]. Among the commercial Pb-free alloys, Sn-58 wt% Bi eutectic alloy may be a favorable alloy specially for electronics and telecommunications. In fact, this alloy, which has the eutectic temperature of 139˚C, has a higher ultimate tensile stress and shear strength than Sn-Pb eutectic [2,3]. Bismuth has also been used as the alloying element in ternary Sn-Zn-Bi [4] Sn-Ag-Bi [5,6] and Sn-BiCu [7] systems to provide suitable substitutes for Sn-Pb solder alloys.
In an effort to develop lead-free solders, most research up to now focused on melting points and physical strengths of alloys, and there has been little consideration of the corrosion properties of base alloys for lead-free solders [8-10]. Moreover, the properties of these Sn-Bi-Sb lead-free alloys in corrosive environments have not been reported, and most of the fluxes are chloride or fluoride base compounds. As a result, most of the corrosion associated with soldered joints is due to the use of improper or insufficient cleaning methods to remove the fluxes [11]. Consequently, the objective of this preliminary study is to investigate the effects of the addition of Sb on structure, melting, corrosion and mechanical properties of SnBi eutectic solder alloys.
2. Experimental Procedure
2.1. Materials and Processing
The materials used were three Sn-58% Bi alloys, containing 0, 3 and 6 wt% Sb. The samples were prepared from high purity (99.99%) tin, bismuth and antimony metals, melted in an electrical furnace under inert argon atmosphere and cast into slabs in alumina crucibles. The chemical composition of the produced alloys was determined by Atomic Absorption Spectrometry (Table 1).
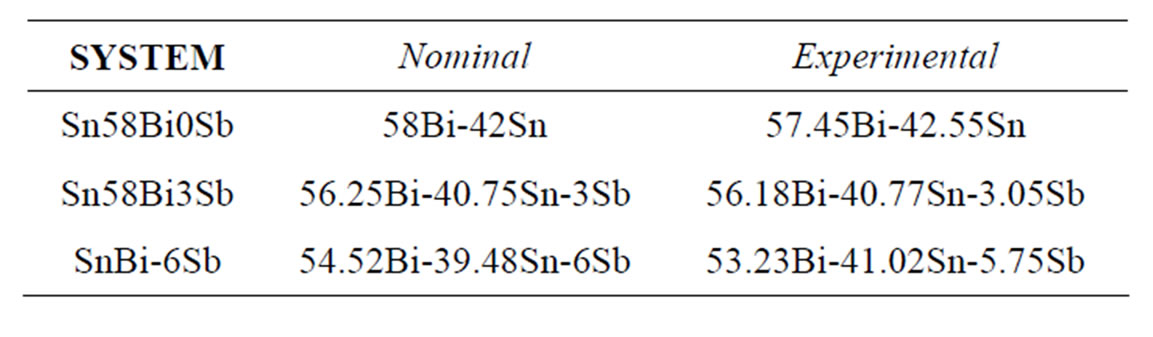
Table 1. Chemical composition of manufactured alloys (wt%).
2.2. Thermal, Structural, Mechanical and Electrochemical Characterization
The thermal behavior of the alloys was investigated using a Perkin-Elmer differential scanning calorimeter (DSC). The specimens with an approximate weight of 50 mg were heated to 300˚C, under inert argon atmosphere, with a constant rate of 10˚C/min. The structure of the alloys was examined by X-ray diffraction (XRD) using a Rigaku diffractometer with CuK radiation (λ = 1.54056 A). A scanning electron microscope (SEM) model Philips XL-30 was used for microstructural analysis and energy dispersive X-ray spectroscopy (EDX) was performed in the SEM to analyze the chemical composition of the alloys. The compressive tests were conducted at room temperature using a Shimadzu AG-I universal testing machine with a constant cross-head speed of 0.5 mm/min. Vickers hardness (VH) was measured using a Vickers microhardness tester.
The specimens were polished with grade 600 and 1000 carborundum papers, rinsed with distilled water and acetone and dried with hot air, previously to the electrochemical tests. The open circuit corrosion potential (Ecorr) of all specimens was recorded before the potentiodynamic polarization measurements using a saturated calomel electrode (SCE) as reference. It was used a cell for flat samples, with a contact area of 1 cm2 between working electrode and electrolyte (a 3% NaCl solution). The cell contained a great superficial area platinum electrode as the counter electrode, and the reference electrode already mentioned. Potentiodynamic polarization measurements were conducted with a scan rate of 10 mV/min. Cathodic potentiodynamic scans were carried out first, starting at the Ecorr value to around –1200 mVsce. Anodic scans were done, after a return to steady-state conditions, from the Ecorr value to around 500 mVsce. All electrochemical measurements were carried out at room temperature and with no de-aeration of the electrolyte.
3. Results and Discussion
3.1. Phases and Microstructure
Figure 1 shows the corresponding Sn-Bi, Bi-Sb and Sn-Sb binary phase diagrams. The Sn-Bi system displays a eutectic reaction with 43 atomic% of bismuth (58 wt%) at a temperature of 139˚C. In contrast, the Bi-Sb system freeze to form a continuous series of solid solutions, contrary to the Sn-Sb system where the tin rich zone exhibits a peritectic reaction and limited solid solution of Sb.