Functional Starter Cultures for Meat: A Case Study on Technological and Probiotic Characterization ()
1. Introduction
The fermentation is one of the oldest forms of food processing and preservation in the world [1] ; most traditional fermentations are conducted as uncontrolled and unpredictable spontaneous processes. Nowadays, some fermented products are produced on a large scale as a result of the application of modern technology, automation in production engineering, and biotechnology in the genetic manipulation of functional microorganisms [1] . The bacteria which have an important role and commonly found in fermented sausages are lactic acid bacteria (LAB), as they are used as starter cultures and promote meat fermentation [2] . A starter culture can be defined as a microbial preparation of large number of cells of at least one microorganism to be added to a raw material and produce a fermented food, conducting its fermentation kinetic and assuring food safety, shelf-life, technological and economic feasibility criteria [3] . Fermentation, conducted by LAB, has been shown to have not only pre- servative effects and the ability of aiding the modification of the physicochemical properties of different foods, but also the capability to provide significant impact on the nutritional quality and functional performances of the raw material [4] . Thus, they have a crucial role in the development of the nutritional, organoleptic, and micro- biological quality, and health improvement of fermented products; in fact, the industrialization of food tech- nology increased the economical importance of lactic acid bacteria, because this offers a possibility to explore the use of lactic acid bacteria as functional starter cultures for the manufacture of fermented foods [5] . Never- theless, small-scale productions continue to use the traditional method of spontaneous fermentation without the addition of starter cultures, and let the “house-flora” to guide the fermentation [6] . Today, the main challenge in the design of starter cultures is to select strains able to improve food safety, and to preserve the typical sensory quality of traditional sausages [7] . The most commonly identified LAB species in traditional sausages are Lac- tobacillus sakei, Lb. curvatus and Lb. plantarum [7] . Lactobacillus strains isolated from fermented products are generally recognized as safe (GRAS, or QPS-qualified presumption of safety―in European Union).
Nowadays, there is a considerable interest towards probiotics for a variety of medical conditions, and millions of people around the world consume probiotics daily to maintain well-being [5] . Probiotics have a positive eco- nomic impact in Developing Countries, in which people suffer from frequent gastrointestinal infections [8] . Specific strains of Lactobacillus, Bifidobacterium and some Propionibacterium strains have been introduced as probiotics in food products due to their health-promoting effects [8] . The criteria for the selection of probiotics include the lack of pathogenicity, the tolerance to gastrointestinal conditions (acid and bile), the ability to adhere to gastrointestinal mucosa and the competitive exclusion of pathogens [8] . According to the FAO/WHO [9] , one of the main selection criteria for potential probiotics strains is that they should adhere to intestinal mucosa and/ or epithelial cells. A new frontier goal for fermented meat is the use of functional starter cultures, i.e. starter cultures with an “added function”; some authors [6] [7] proposed the use of probiotic strains in sausages.
The selection of a starter is a complex process, involving different steps, like a preliminary characterization under laboratory conditions, the selection of the most promising strains, a lab validation and the final validation in a large-scale fermentation; the selection of a functional starter includes a further step, dealing with the assess- ment of the functional traits [10] . This paper proposes a case study on how to use a step-by-step approach, based on some rapid protocols, to select promising starter cultures with probiotic abilities; some strains isolated from a commercial starter were used as targets. The performances of the targets were compared with those experienced by some collection isolates.
2. Materials and Methods
2.1. Strains
Different strains were used throughout this study:
Nine strains of lactic acid bacteria (labeled from st1 to st9), isolated from a commercial preparation and identified as Lb. sakei, using the approach proposed by Bevilacqua et al. [11] ;
Five strains purchased from a Public Collection (DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen’s collection, Braunschweig, Germany): Lactobacillus curvatus subsp. curvatus DSMZ 20019, Lb. plantarum DSMZ 1055, Lb. sakei DSMZ 15831, Leuconostoc carnosum DSMZ 5576, Pediococcus acidilactici DSMZ 20284.
Lb. plantarum c19, a promising probiotic strain [11] .
The strains were stored at −20˚C in MRS broth (Oxoid, Milan, Italy) added with 33% of sterile glycerol (J.T. Baker, Milan, Italy). Before each assay, the strains were grown under anaerobic conditions in MRS broth, incubated at 37˚C for 24 h.
2.2. Preliminary Characterization and Metabolism
The isolates from the commercial starter were characterized for the following traits:
a) Hetero-fermentative activity. It was carried out in MRS broth, supplemented with glucose (2%) (JT Baker, Milan) and inoculated to 7 log cfu/ml. Gas production from glucose was determined using a Durham tube, after the incubation at 30˚C for 7 days.
b) Hydrolysis of arginine. The ability to hydrolyze arginine with the production of ammonia and carbon dioxide was verified in the substrate of Abd-El-Malek (5 g/l tryptone, 2.5 g/l yeast extract, 0.5 g/l glucose, 2 g/l K2HPO4, 3g/l arginine hydrochloride); the pH was adjusted to 7.0. The broth was inoculated to 7 log cfu/ml and incubated at 30˚C for 96 h. The presence of ammonia was detected qualitatively by the addition of one drop of Nessler’s reagent (C. Erba, Milan, Italy).
c) Hydrolysis of aesculin. LAB were inoculated in MRS broth, buffered to pH 6.5 and supplemented with esculin (2 g/l) (Sigma-Aldrich) and ferric ammonium citrate (1 g/l) (C. Erba). The samples were then incubated at 30˚C for 72 h; colour turning to black denoted aesculin hydrolysis.
d) Production of acetoin. The production of acetoin was evaluated in the following broth: 5 g/l bacteriological peptone, 5 g/l glucose, 5 g/l K2HPO4. The broth was inoculated with LAB to 7 log cfu/ml and incubated at 30˚C for 4 - 7 days. Colour turning to red after the addition of a drop of a 6%-α-naphthol solution (Sigma Aldrich) and a drop of 16% aqueous solution of NaOH highlighted the production of acetoin.
e) Reduction of nitrates. This test was performed in the Nitrate broth (8.6 g/l of peptone; 6.4 g/l NaCl, 1.5 g/l KNO3); the pH was adjusted to 7.0 - 7.2. The samples were inoculated to 7 log cfu/ml and incubated at 30˚C for 72 - 96 h. The reduction of nitrate to nitrite was detected qualitatively by the addition of Griess Ilosvay’s reagent (C. Erba).
f) Slime production. The production of slime was tested on MRS Agar (Oxoid) supplemented with 5% sucrose (J. T. Baker). After streaking the strains onto the surface, the plates were incubated at 30˚C for 7 days.
g) Lipolytic activity. This trait was assessed on PCA (Oxoid) supplemented with 2% of tributyrin (Sigma Aldrich). After inoculation, the plates were incubated at 30˚C for 7 days; a clear halo around the colonies highlighted the hydrolysis of tributyrin.
h) Proteolytic activity. The strains were streaked onto the surface of PCA + 5% Skim Milk Powder (Oxoid), incubated at 30˚C for 7 days.
2.3. Acidification
The acidifying ability (i.e. the decrease of pH) was assessed in MRS broth, inoculated to 6 log cfu/ml and incubated at 15˚C, 25˚C, 37˚C and 44˚C; the pH was measured through a pH meter Crison 2001 (Crison Instruments, Barcelona, Spain).
2.4. Effect of Temperature, NaCl, Nitrites and Nitrates on LAB Growth
The assay was performed in MRS broth, added with NaCl (2%, 4%, 6.5%, 8%), nitrates (NaNO3, 100 to 500 ppm), nitrites (NaNO2, 50 to 300 ppm). The samples were inoculated to 6 log cfu/ml and incubated at 10˚C, 15˚C, 25˚C, 30˚C, 37˚C and 44˚C up to 7 days. Aliquots of not modified MRS broth (pH 6.2), inoculated with the LAB and incubated at 30˚C, were used as positive controls. Microbial growth was evaluated as absorbance at 600 nm, using a spectrophotometer Shimadzu UV-visible 1601 model 1642 (Shimadzu Europe Ltd., Duisburg, Germany). Data were modelled as Growth Index (GI) [12] :
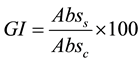
where Abss is the absorbance of the samples at different NaCl concentrations, nitrates and nitrites concentrations, and temperature, and Absc the absorbance of the positive control.
2.5. Auto-Aggregation Assay
Auto-aggregation was determined as described by Collado et al. [8] . Briefly, bacteria were grown for 18 h at 37˚C in MRS broth (Oxoid); then cells were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS 1 M; Sigma-Aldrich). The absorbance of the suspension (600 nm) was adjusted to 1.0; afterwards, 10 ml of suspension were placed in sterile tubes and incubated at 25˚C, and the absorbance at 600 nm of the upper suspension was monitored after 2, 4, 6, 12, 24 h. Auto-aggregation percentage was calculated with the following formula:
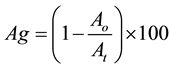
where Ao and At are respectively the initial value and the absorbance at the time t.
2.6. Hydrophobicity Assay
The experiment was carried out according to the method described by Gong et al. [5] and Perricone et al. [13] , with some modifications. Briefly, bacteria were grown at 37˚C in MRS broth (Oxoid); then, cells were harvested by centrifugation and washed twice with PBS buffer 0.1 M. 9.5 ml of cell suspension were added with 0.5 ml of hexadecane or xylene (Sigma-Aldrich), thoroughly mixed for 30 s and left under static conditions for 10 min. The ability of hexadecane to catch cells was evaluated through absorbance measurement at 600 nm after 60, 120, and 240 min and the data were modeled as Hydrophobic Index [13] .
2.7. Co-Aggregation Assay
The co-aggregation test was performed as described by Collado et al. [8] . For this assay, the pathogenic strains were: Escherichia coli O157:H7 and Salmonella sp., belonging to the Culture Collection of the Laboratory of Predictive Microbiology, University of Foggia Briefly, bacteria suspensions were prepared as described for auto-aggregation analysis. Pathogens were grown in Nutrient broth (Oxoid) at their optimal conditions.
Equal volumes of cell suspension of each LAB (ca. 8 log cfu/ml) and pathogen strains (8 log cfu/ml) (1:1) were mixed for 20 s and then incubated at 25˚C and the absorbance at 600 nm was evaluated immediately after the preparation and after 2, 4, 6, 12, 24 h; two different controls were prepared: a sample containing only LAB and a sample with pathogens. Data were modeled as follows:
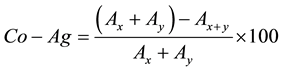
where Ax and Ay represent the absorbance of LAB and pathogens, respectively, and A(x+y) is the absorbance of the mix.
2.8. Survival at pH 2.5 and with 0.3% of Bile Salt Added
Bacteria were grown at 37˚C in MRS broth for 24 h; cells were harvested by centrifugation and suspended in sterile distilled water. Aliquots of distilled water, inoculated with each LAB separately (7 - 8 log cfu/ml) acidified to pH 2.5 or added with bile salt 0.3% (w/v) (Oxoid) were stored at 37˚C. Cell viability was evaluated after 3 and 24 h through the pour plating (MRS agar incubated at 30˚C for 2 - 4 days under anaerobic conditions). Aliquots of distilled water inoculated with the strains were used as controls.
2.9. Antibiotic-Resistance
Antibiotic-resistance was carried out with the Kirby-Bauer method. The antibiotics used were: Erythromycin (78 mg), Gentamicin (40 mg), Chloramphenicol (60 mg), Tetracyclines (80 mg) Ampicillin (33 mg), Trimethoprim (5.2 mg), Ciprofloxacin (10 mg), Vancomycin (70 mg) (Neo SensitabsÒ, Taastrup, Denmark).
2.10. Statistical Analysis
All the analyses were performed in duplicate over two different batches; the results were analyzed through one- way analysis of variance (one-way ANOVA), using the Tukey’s test as the post-hoc comparison test (P < 0.05). Moreover, the data from the technological and the probiotic characterization were used as input value to run a Principal Component Analysis. The statistical analyses were performed through the software Statistica for Windows (Statsoft, Tulsa, Okhla.).
3. Results and Discussion
3.1. Technological Characterization
The strains from the commercial preparation possessed an homofermentative metabolism and produced ammonium from arginine, whilst they did not hydrolyze the aesculin. Concerning the other enzymatic activities, they showed the production of slime (except for the strains st1 and st4), but they did not possess proteolytic and lipolytic metabolism (data not shown).
The study of the phenotypic and technological characteristics was a fundamental and critical step to define the profile of a meat starter. Heterofermentative LAB are not suitable for sausages production, because the formation of large amounts of carbon dioxide leads to holes of different sizes in the product; in addition, these LAB produce high acid acetic concentration that causes a pungent off-flavour [14] . Thus the homo-fermentative metabolism is a fundamental trait for a sausage starter and the isolates from the commercial preparation fulfill this requirement. Concerning the other enzymatic activities, the wild isolates were able to hydrolyze arginine and this trait was recovered in some other strains proposed as starter cultures for meat [15] [16] . The results for the proteolytic and lipolytic activities are in line with the results of many authors, as LAB do not possess significant proteolytic or lipolytic patterns, although a certain degree of peptidase and lipase activity was recovered in some strains isolated from meat products [6] [7] .
Figure 1 shows the acidification of MRS after 24 h; the strains from the commercial starter decreased the pH at 15˚C by 0.7 and experienced a higher ΔpH (ca. 1.5) at 25˚C, whilst the collection isolates did not decrease the pH at 15˚C.
Concerning the effect of the temperature (Figure 2), as expected after 24 h none of the studied strains (both the isolates from the commercial preparation and the collection microorganisms) showed a significant growth at 10˚C and 15˚C, whereas at 44˚C the GI was strongly strain-dependent, being ca. 100% for the collections strains and for 3 isolates from the commercial starter (st3, st6 and st8) and ca. 0% - 5% for the isolates st1, st2, st4, st5, st7 and st9. After 48 h (Figure 2(b)), we found a significant increase of the GI at 15˚C for all the isolates from the commercial starter and for some collection strains (Ln. carnosum, P. acidilactici, Lb. plantarum
![]()
Figure 1. Acidification of the isolates from the starter and the collection strains in MRS broth, stored at 15˚C, 25˚C, 37˚C and 44˚C for 24 h. Data are the mean value. A, Ln. carnosum; B, P. acidilactici; C, Lb. curvatus; D, Lb. plantarum DSMZ 1055; E, Lb. plantarum C19; F, Lb. sakei.
![]() (a)
(a)![]() (b)
(b)
Figure 2. Growth Index (%) of the strains in MRS broth incubated at different temperatures after 24 (a) and 48 h (b). Data are the mean value. A, Ln. carnosum; B, P. acidilactici; C, Lb. curvatus; D, Lb. plantarum DSMZ 1055; E, Lb. plantarum C19; F, Lb. sakei.
c19 and Lb. sakei); moreover, the isolates st1, st3, st4, and st5 showed a GI > 25% at 10˚C (ca. 27% - 32%), which pointed out a moderate growth and only a partial inhibition due to the low temperature [17] . After 48 h there was also a significant increase of the GI at 44˚C for the isolates st4, st5, and st9.
Figure 3 reports the effect of salt; the isolates st1 to st9 were moderately affected by 4% NaCl, with a GI of 45% - 66%, and were strongly inhibited by higher amounts; after 48 h there was a significant increase of GI in the broth containing 6.5% of NaCl up to 50% - 60% (data not shown).
The effect of temperature on the isolates from the commercial preparation was in line with the results of many authors. For example, Papamonoli et al. [15] and Santos et al. [18] [19] reported that ca. 80% - 90% of LAB strains from meat were able to grow at 15˚C, whilst the ability to grow at higher temperatures was found only in a half of meat microbiota [16] . Concerning the effect of salt, our strains showed a significant deviation from the expected outcome, as they appeared more sensitive than the wild microbiota of meat able to grow in presence of 6.5% - 10% NaCl [15] .
![]()
Figure 3. Growth Index (%) of the strains in MRS broth + NaCl after 24 h. Data are the mean value. A, Ln. carnosum; B, P. acidilactici; C, Lb. curvatus; D, Lb. plantarum DSMZ 1055; E, Lb. plantarum C19; F, Lb. sakei.
3.2. Functional Characterization
The functional characterization was based upon the assessment of some classical traits (survival at pH 2.5 and with bile salts added, antibiotic resistance), as well as on some screening protocols for the evaluation of the ability to adhere to the mucosa of the gut (hydrophobicity, auto-aggregation) and the antimicrobial activity towards food-borne pathogens (co-aggregation).
Based on the Hydrophobic Index (Figure 4), the different targets could be divided into different groups as follows:
a) Strains with a hydrophilic trend (low Hydrophobic Index, HI) or with a variable HI: st2, st6, st8, st9, Lb. curvatus;
b) Isolates with a moderate HI (ca. 16%), st1, st3, st4 and st5;
c) Collection strains with a high HI, Ln. carnosum, P. acidilactici, and both the strains of Lb. plantarum;
d) Lb. sakei showed an intermediate trend between the isolates from the commercial preparation and the collection strains.
Another interesting trait is the auto-aggregation (Table 1) (Ag); this parameter increased over the time and after 24 h all the targets showed a Ag > 32%, with the maximum values recovered for the isolates st9 and Ln. carnosum.
The targets were able to exert a significant bioactivity towards Salmonella sp. after 12 h, with a co-aggrega- tion index (Co-Ag) ranging from 21.40% (isolate st2) to 42.99% (Lb. curvatus); the statistical analysis (one-way ANOVA) highlighted a continuous distribution rather than a discrete grouping of the strains, being the less active the strain st2 and the most effective the collection strain of Lb. curvatus, whilst all the other strains belonged to an intermediate class of bioactivity (Co-Ag of 24.56% - 38.04%) (Table 2). On the other hand, the microorganisms experienced lower Co-Ag towards E. coli, with an index up to 17% (data not shown).
Concerning the effect of bile salt, the targets experienced a low reduction in cell count (up to 1.76 log cfu/ml for the isolate st6), whilst the acidic pH exerted a stronger effect, being the strains st6 and Lb. curvatus the most sensitive ones, as they were respectively reduced by 5.79 and 3.50 log cfu/ml, against the viable count of the other strains decreasing by 1 - 2 log cfu/ml (Figure 5).
The final step of the probiotic characterization was the evaluation of the antibiotic-resistance. All the strains were resistant to vancomycin, ciprofloxacin and trimethoprim, while for the other antibiotics the bacterial growth was inhibited, with the exception of the isolates st3 and st1, because they were resistant to Gentamicyn and Tetracyclines, respectively (data not shown).
Auto-aggregation and hydrophobicity play an important role in the adhesion to gut mucosa [5] . Bacterial
![]()
Figure 4. Hydrophobic Index (%) of the strains. Mean value ± standard deviation. The small letters indicate the significant differences (one-way ANOVA and Tukey’s test). A, Ln. carnosum; B, P. acidilactici; C, Lb. curvatus; D, Lb. plantarum DSMZ 1055; E, Lb. plantarum C19; F, Lb. sakei.
![]()
Table 1. Auto-aggregation of the targets after 24 h; mean values ± standard deviation. Data were analyzed through one-way ANOVA, using the approach of the homogeneous group.
![]()
Figure 5. Decrease in cell count after 3 h of incubation in distilled water acidified to pH 2.5 or added with bile salt. Mean values ± standard deviation.
![]()
Table 2. Co-aggregation of LAB with Salmonella sp.: mean values ± standard deviation. Data were analyzed through one- way ANOVA, using the approach of the homogeneous group.
adhesion to intestinal epithelial cells depends by composition and structure of the membranes and force of interaction [8] [20] ; moreover, it is a multistep process, connected to the hydrophobic properties of cell surfaces. It has been reported that the most hydrophobic a microorganism behaves the most it adheres to gut [13] .
Another desired trait for a probiotic is the ability to compete with pathogens in the intestine; we studied this phenomenon through the co-aggregation. It is an indirect index connected with the ability to catch pathogens, thus avoiding their adhesion to the mucosa [8] .
A final highlight on the implication of antibiotic resistance. The antibiotic resistance per se is not a safety issue, but it could pose a strong threat for the health when the genetic determinants are carried out by mobile elements [21] . Therefore EFSA (European Food Safety Authority) requires for a food-grade microorganism to be sensitive to the antibiotics or at least to possess chromosomal determinants for the resistance [22] .
For this last trait, the profile of the isolates from the commercial starter was in line with the literature [7] [22] , with two exceptions to this statement: the isolates st1 and st4.
3.3. Multivariate Analysis
The data from the technological characterization, as well as the probiotic traits of the targets, were used as input data to run a multivariate analysis; the targets could be divided into 2 main groups, the first one comprising the collection strains and the second one the isolates from the commercial starter; however, there were some exceptions to this generalized statement, as Lb. sakei showed an intermediate trend between the collection and the wild isolates and Lb. plantarum c19 experienced a trend different from all the other collection strains (Figure 6(a) and Figure 6(b)).
![]() (a)
(a)![]() (b)
(b)
Figure 6. Principal component analysis (PCA); the results of technological and probiotic characterization were used as input data. (a) Projection of the variables; (b) Multivariate isolates.
The isolates from the starter showed better performances at lower temperatures (10˚C and 15˚C) in terms of growth and acidification of a lab medium, with some interesting values of co-aggregation towards E. coli O157:H7; on the other hand the collection isolates showed interesting functional traits and better performances in presence of salt and at higher temperatures.
4. Conclusions
This paper shows a case study on a possible flow-sheet with some rapid protocols to select promising starter cultures, with probiotic abilities, intended for meat. The selection relies upon some traits, i.e.:
a) The qualitative assessment of the enzymatic activities;
b) The evaluation of the performances (acidification) and growth by using the Growth Index approach;
c) A screening on the probiotic properties, using some classical traits (antibiotic resistance, resistance at low pH and in presence of bile salts) and some indirect indices connected with the adhesion to the mucosa (aggregation and hydrophobicity) and the bioactivity towards pathogens (co-aggregation);
d) The selection of the most promising strains or the evaluation of the global performance of the strains through a multivariate approach.
Concerning the performances of the isolates from the commercial starter, they experienced the classical ability of the starter culture intended for meat and were able to perform the fermentation at low temperatures, with some interesting traits in terms of bioactivity towards E. coli O157:H7.
Acknowledgements
This research was supported by the Italian Ministry of Education, University and Research (MIUR) through the project PON-01-1409 “Process and product innovations aimed at increasing food safety and at diversifying pork-based products” (SAFEMEAT).
NOTES
*Corresponding author.