Open Journal of Modern Neurosurgery
Vol.08 No.03(2018), Article ID:86172,11 pages
10.4236/ojmn.2018.83028
Traumatic Acute Subdural Hematoma: Treatment by Evacuation with Decompressive Craniotomy and Cranioplasty, Case Series and Surgical Outcome Analysis
Ahmed. M. Elshanawany*, Abdelhakeem A. Essa
Department of Neurosurgery, Faculty of Medicine, Assiut University, Assiut, Egypt

Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: May 31, 2018; Accepted: July 22, 2018; Published: July 25, 2018
ABSTRACT
Background: Acute subdural hematoma (ASDH) is considered the most common traumatic brain mass lesion. Its prognosis is still grave despite the improvements in treatment modalities. Its mortality rate was reported to be around 60% until the 1990s. In the last decade, ASDH mortality rate was reduced to the level of 20% - 40%. Standard treatment to decrease intracranial tension via hematoma evacuation is associated with decompressive craniotomy and followed by ICU management. Objective: To evaluate the outcome and prognostic factors in patients of acute subdural hematoma treated by surgical evacuation and decompressive craniotomy. Also, outcome of cranioplasty by repositioning of patients own bone or by synthetic mesh methods is evaluated. Patients and Methods: It is one year retrospective study. It was conducted on 53 patients, in trauma unit, Assiut university hospitals. We report time lag between trauma and performed surgery, initial Glasgow coma scale (GCS), age, sex and presence of other intracranial pathologies. Outcome assessment is based on Glasgow outcome scale (GOS) and follow-up extended for 6 months. We include those patients with only (isolated) head trauma, shift of midline more than 5 mm in CT brain. We excluded patients with GCS 3 and fixed dilated pupils as well as patients with GCS higher than 12. We did decompressive craniotomy and duraplasty in all patients. Bone flap of decompressive craniotomy is situated in the abdomen. All functionally recovered patients were submitted for cranioplasty with either replacing patient own bone or by Titanium mesh. Results: We had 39 males and 14 females. Age ranged between 7 and 65 years old. 23 deaths, 10 persistent vegetative state, 10 severe disability, 8 moderate disability and 2 good recovery. The outcome analysis was based on 6 month follow-up. Conclusion: Acute subdural hematoma is a very serious condition. Mortality and morbidity is intimately related to GCS on admission. Presence of associated cerebral pathology increases mortality and morbidity of patients with posttraumatic acute subdural hematoma. Early evacuation of posttraumatic acute subdural hematoma with decompressive craniotomy is an important method to control raised intracranial tension, reduce shift of midline and very beneficial in decreasing mortality and morbidity. Regarding infection and avoiding bone flap resorption, Titanium mesh is better than patient own bone during cranioplasty after patient recovery.
Keywords:
Acute Subdural Hematoma, Hematoma Evacuation, Decompressive Craniotomy, Cranioplasty

1. Introduction
Acute subdural hematoma (ASDH) is an important sequelae of traumatic brain injury. It is represented in one third of severe head injuries [1]. Despite the development in intensive care management and neurosurgical techniques, traumatic acute subdural hematoma still carry high morbidity and mortality [2]. Mortality rate increases up to 60% of cases [1]. In the western world mortality rate may be better but still reporting that ASDH carries an important role in mortality under 45 years old [3]. Hemorrhage in the subdural space is due to rupture of bridging veins between dura and cortex, rupture of venous sinuses and injury of cortical arteries and veins [4]. Hematoma leads to increase intracranial tension and changes in the cerebral perfusion. Hematoma is usually associated with other parenchymal pathologies as subarachnoid hemorrhage, cerebral contusions and brain edema. Many prognostic factors may affect on patients outcome as patients’ age, conscious level of the patient on admission (Glasgow coma scale), time lag between injury and surgical care and associated brain injuries [5]. Toutant et al., in a study of 218 patients with a mortality rate of 77% supposed that the proposed prognostic factor is absence of basal cisterns [6]. Kim et al., in a study of 256 patients with a mortality rate of 38.8% supposed the proposed prognostic factors are admission GCS, mechanism of injury, pupillary abnormalities and thickness of the hematoma [7]. Bartels et al., in a study of 59 patients supposed the proposed prognostic factor is size of hematoma and midline shift [8].
Our study is aiming to detect and introduce the outcome and prognostic factors in patients of acute subdural hematoma treated by surgical evacuation and decompressive craniotomy based on our observations.
2. Patients and Methods
It is one year retrospective study. It was conducted in the period between Jan 2017 to Jan 2018, on 53 patients, in trauma unit, Assiut university hospitals. We report initial GCS, age, sex, presence of other intracranial pathologies and time lag between time of trauma and onset of surgery. CT brain on admission was assessed for hematoma thickness and amount of midline shift. Outcome assessment based on Glasgow outcome scale (GOS) and follow up extended for 6 months.
Glasgow outcome scale categorize patients outcome as follows: Death: for patients who died, vegetative state which defined as patients that lost intellectual function of the brain and has minimal responsiveness, severe disability: conscious patients but depends on others for daily support, moderate disability: who is disabled but independent; can work in sheltered setting and good recovery: for patients that resume nearly normal life [9].
We include those patients with only (isolated) head trauma, hematoma thickness greater than 10 mm and shift of midline more than 5 mm in CT brain. We exclude polytraumatized patients and those with acute subdural hematoma because of non traumatic causes. Also we excluded patients with hematoma thickness less than and shift of midline less than 5 mm. We excluded patients with GCS 3 and fixed dilated pupils as well as patients with GCS higher than 12 provided there is no unequality of pupils.
We did wide skin flap, large decompressive craniotomy and duraplasty in all patients. Surgery was done as early as patient was admitted to our trauma unit. Bone flap of decompressive craniotomy was placed in the abdomen. After 6 months of the insult, all functionally recovered patients were submitted for cranioplasty with Titanium mesh.
3. Results
53 patients with traumatic acute subdural hematoma were operated upon. We performed hematoma evacuation and decompressive craniotomy in all patients. The removed bone was placed subcutaneously in the abdomen. We had 39 (73.5%) males and 14 (26.5%) females (Table 1). Age ranged between 7 and 65 years old with mean 35.3 (Table 2). Outcome was evaluated based on Glasgow outcome score (GOS); 23 deaths (43.4%), 10 persistent vegetative (18.87%) state, 10 severe disability (18.87%), 8 moderate disability (15.1%) and 2 good recovery (3.76%) (Chart 1). On admission GCS ranged between 4 and 11.Regards time lag between onset of trauma and performed surgery, it was ranged between 3 hours and 18 hours (Table 3 and Table 4).
We had 6 patients (11.3%) were admitted with GCS 4, all of them died. 2 patients (3.77%) were admitted with GCS 11, both of them showed good recovery (Table 5). Both patients that showed good recovery were operated within 5
Table 1. Sex distribution.
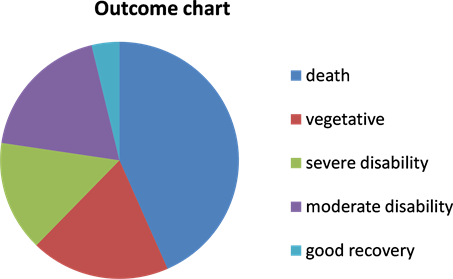
Chart 1. Outcome chart.
Table 2. Demographic data.
Table 3. Outcome according to sex distribution.
Table 4. Outcome according to age.
Table 5. Outcome according to GCS.
hours of onset of trauma. All patients that showed moderate disability were operated within 5 hours after the onset of trauma. For those patients with severe disability, 4 patients were operated within 5 hours after onset of trauma and 6 patients after 5 hours of onset of the trauma. For vegetative patients, 3 patients were operated within 5 hours after onset of trauma and 7 patients were operated after 5 hours of onset of the trauma. Patients who died, 10 patients were operated within 5 hours of trauma and 13 patients were operated after 5 hours of trauma (Table 6).
We reported presence of additional brain pathologies. We considered brain edema is a common association with ASDH and is not an additional brain pathology. 8 patients were with isolated ASDH and underlying edema and 45 patients were with additional pathologies as depressed skull fractures (DF) in 7 patients, intraventricular hemorrhage (IVH) in 19 patients, subarachnoid hemorrhage (SAH) in 24 patients and cerebral contusisons (CC) in 35 patients (Table 7 and Table 8). According to mechanism of trauma; we had 20 (37.5%) patients due to road traffic accidents (RTA), 18 patients (34%) due to assault from others (AFO) and 15 patients due to falls from height (FFH) (28.5%) (Table 9).
For 20 patients who functionally recovered (not vegetative), we performed cranioplasty. We prefer to do cranioplasty not before 3 months after onset of trauma. Cranioplasty was by repositioning patient own bone in 6 cases and by using Titanium mesh in 14 patients. Bone flap resorbtion was found in 7 patients.3 patients of those had cranioplasty by their own bone got infected. We removed the infected bone and cranioplasty were redone by using Titanium mesh, 3 months later (Table 10) (Figure 1 and Figure 2).
4. Discussion
Traumatic ASDH is an important entity because of its high mortality and morbidity. It represents about 30% of brain injuries [10]. Its mortality rate was reported to be around 60% until the 1990s. In the last decade, ASDH mortality rate was reduced to the level of 20% - 40% [11] [12]. In traumatic ASDH, the
Figure 1. Postoperative image, after hematoma evacuation and decompressive craniotomy.
Figure 2. Intraoperative image showing cranioplasty with Titanium mesh.
Table 6. Outcome regards time lag between admission and surgery.
Table 7. Outcome regards associated posttraumatic pathologies.
DF: Depressed Fracture; IVH: Intraventricular Hemorrhage; SAH: Subarachnoid Hemorrhage; CCs: Cerebral Contusions.
Table 8. Summary of associated posttraumatic pathologies.
DF: Depressed Fracture; IVH: Intraventricular Hemorrhage; SAH: Subarachnoid Hemorrhage; CCs: Cerebral Contusions.
Table 9. Outcome regards cause of trauma.
RTA: Road Traffic Accident; AFO: Assault from Others; FFH: Falls From Height.
Table 10. Summary of cranioplasty results.
functional recovery rate ranges between 19% and 45% [13]. Acute subdural hematoma occurs in of 3 mechanisms: damage to surface cortical vessel, bleeding from underlying parenchyma injury and tearing of bridging veins from cortex to dural venous sinuses [14]. Although the indications for operation have not been clearly established in traumatic ASDH, hematoma evacuation and decompressive craniectomy were performed to decrease intracranial tension [15].
Yanagawa et al. reported that 67%of the patients of traumatic ASDHs were male and the mean age was 43 years [16]. In our study, males represent 73.5% and mean age was 36. GCS on admission is the most important factor that directly reflects brain damage, reflects clinical status, and the considered prognosis [16]. In our study patients with GCS below 8 showed mortality rate 63.89% and outcome becomes better as the GCS improves. Early surgery helps to improve outcome and decrease mortality and morbidity.
In our study, we evaluated functional outcome on bases of Glasgow outcome score. Glasgow outcome score is composed of five components. Those five components are death, vegetative state, severe disability, moderate disability and good recovery. In our study, mortality rate was 43.4%. Those patients who recovered with moderate functional disability represent 15.1%. Severe disability represents 18.7%. Vegetative patients represent 15.1%.
Both Ryan et al. and Leitgeb et al. reported that the most common cause of ASDH is falls from height the road traffic accidents [11] [17]. In our study, the most common cause of ASDH is road traffic accidents followed by falls from height and finally assault from others. Leitgeb et al. demonstrated a higher mortality rate in patients with additional traumatic brain lesions [17]. In our study, we found ASDH with other brain pathologies represent about 85% of cases. We considered association of brain edema in the area underlying the hematoma is usual association and not an associated lesion. In our study, mortality rate increases in presence of posttraumatic additional cerebral pathology. In cases with associated IVH, mortality rate reaches 35.8%. In cases with associated CCs, mortality rate reaches 66% and in those with SAH, mortality rate reaches 45.3%. In cases with isolated ASDH, mortality rate reaches 12.1%.
Cranioplasty for those patients that recover is important to protect brain, achieve normal appearance and avoid sinking skin flap syndrome. Best time for cranioplasty after decompressive craniotomy is debatable. Some authors define early cranioplasty as that done before 3 months of decompressive craniotomy [18]. Delay in cranioplasty timing aims to decrease possibility of infection. Some consider cranioplasty before 6 months after decompressive craniotomy carries a poor outcome [19]. In our study we perform cranioplasty not before 3 months after trauma to ensure patients recovery and decrease risk of infection. In our cases, resorbtion of bone flap was found in 7 patients. An experimental study on cranioplasty with patient own bone flap also showed that half of the flaps were resorbed [20]. We found less risk of infection with Titanium mesh than bone flap. Preservation of bone flap in the subcutaneous tissue of abdomen made it more susceptible for infection.
We consider this study has some limitations in form of number of patients and include more prognostic factors in our analysis.
5. Conclusion
Acute subdural hematoma is a very serious condition. Mortality and morbidity is intimately related to GCS on admission. Presence of associated cerebral pathology increases mortality and morbidity of patients with posttraumatic acute subdural hematoma. Early evacuation of posttraumatic acute subdural hematoma with decompressive craniotomy is an important method to control raised intracranial tension, reduce shift of midline and very beneficial in decreasing mortality and morbidity. Regarding infection and avoiding bone flap resorption, Titanium mesh is better than patient own bone during cranioplasty after patient recovery.
Funding
We did not receive any fund form any organization or persons.
Conflict of Interest
There is no conflict of interest as this paper is not submitted for publication in any other journal.
Ethical Approval
We in Assiut university hospitals, Faculty of medicine approve all ethical requirements and standards of the journal.
Informed Consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article. As regards the children that were included in this study, informed consent was obtained from parents.
Cite this paper
Elshanawany, A.M. and Essa, A.A. (2018) Traumatic Acute Subdural Hematoma: Treatment by Evacuation with Decompressive Craniotomy and Cranioplasty, Case Series and Surgical Outcome Analysis. Open Journal of Modern Neurosurgery, 8, 331-341. https://doi.org/10.4236/ojmn.2018.83028
References
- 1. Godlewski, B., Pawelczyk, A., Pawelczyk, T., Ceranowicz, K., Wojdyn, M. and Radek, M. (2013) Retrospective Analysis of Operative Treatment of a Series of 100 Patients with Subdural Hematoma. Neurologia Medico-Chirurgica (Tokyo), 53, 26-33.
- 2. Busl, K.M. and Prabhakaran, S. (2013) Predictors of Mortality in Non-traumatic Subdural Hematoma. Journal of Neurosurgery, 119, 1296-1301. https://doi.org/10.3171/2013.4.JNS122236
- 3. Hyder, A.A., Wunderlich, C.A., Puvanachandra, P., Gururaj, G. and Kobusingye, O.C. (2007) The Impact of Traumatic Brain Injuries: A Global Perspective. Neurological Rehabilitation, 22, 341-353.
- 4. Shen, J., Pan, J.W., Fan, Z.X., Zhou, Y.Q., Chen, Z. and Zhan, R.Y. (2013) Surgeryfor Contralateral Acute Epidural Hematoma Following Acute Subdural Hematoma Evacuation: Five New Cases and a Shortliterature Review. Acta Neurochirurgica (Wien), 155, 335-341. https://doi.org/10.1007/s00701-012-1569-9
- 5. Marmarou, A., Lu, J., Butcher, I., McHugh, G.S., Murray, G.D., Steyerberg, E.W., et al. (2007) Prognostic Value of the Glasgow Coma Scale and Pupil Reactivity in Traumatic Brain Injury Assessed Pre-Hospital and on Enrollment: An IMPACT Analysis. Journal of Neurotrauma, 24, 270-280. https://doi.org/10.1089/neu.2006.0029
- 6. Toutant, S.M., Klauber, M.R., Marshall, L.F., Toole, B.M., Bowers, S.A., Seelig, J.M., et al. (1984) Absent or Compressed Basal Cisterns on First CT Scan: Ominous Predictors of Outcome in Severe Head Injury. Journal of Neurosurgery, 61, 691-694. https://doi.org/10.3171/jns.1984.61.4.0691
- 7. Kim, K.H. (2009) Predic-tors for Functional Recovery and Mortality of Surgically Treated Traumatic Acute Subdural Hematomas in 256 Patients. Journal of Korean Neurosurgical Society, 45, 143-150. https://doi.org/10.3340/jkns.2009.45.3.143
- 8. Bartels, R.H., Meijer, F.J., van der Hoeven, H., Edwards, M. and Prokop, M. (2015) Midline Shift in Relation to Thickness of Traumatic Acute Subdural Hematoma Predicts Mortality. BMC Neurology, 15, 220. https://doi.org/10.1186/s12883-015-0479-x
- 9. Jennette, B. and Bond, M. (1975) Assessment of Outcome after Severe Brain Damage. Lancet, 1, 480-484. https://doi.org/10.1016/S0140-6736(75)92830-5
- 10. Murray, G.D., Teasdale, G.M., Braakman, R., Cohadon, F., Dearden, M. and Iannotti, F. (1999) The European Brain Injury Consortium Survey of Head Injuries. Acta Neurochirurgica (Wien), 141, 223-236. https://doi.org/10.1007/s007010050292
- 11. Ryan, C.G., Thompson, R.E., Temkin, N.R., Crane, P.K., Ellenbogen, R.G. and Elmore, J.G. (2012) Acute Traumatic Subdural Hematoma: Current Mortality and Functional Outcomes in Adult Patients at a Level I Trauma Center. Journal of Trauma and Acute Care Surgery, 73, 1348-1354. https://doi.org/10.1097/TA.0b013e31826fcb30
- 12. Meyer, S., Gibb, T. and Jurkovich, G.J. (1993) Evaluation and Significance of the Papillary Light Reflex in Trauma Patients. Annals of Emergency Medicine, 22, 1052-1057. https://doi.org/10.1016/S0196-0644(05)82750-7
- 13. Tallon, J.M., Ackroyd-Stolarz, S., Karim, S.A. and Clarke, D.B. (2008) The Epidemiology of Surgically Treated Acute Subdural and Epidural Hematomas in Patients with Head Injuries: A Population-Based Study. Canadian Journal of Surgery, 51, 339-345.
- 14. Prahaladu, P., Satyavara Prasad, K., Rajasekhar, B. and Satyanarayana Reddy, K. (2017) Clinical Study of Acute Subdural Haematoma—A Level I Trauma Care Centre Experience. International Journal of Research in Medical Sciences, 5, 857-862.
- 15. Li, L.M., Kolias, A.G., Guilfoyle, M.R., Timofeev, I., Corteen, E.A., Pickard, J.D., Menon, D.K., Kirkpatrick, P.J. and Hutchinson, P.J. (2012) Outcome Following Evacuation of Acute Subdural Haematomas: A Comparison of Craniotomy with Decompressive Craniectomy. Acta Neurochirurgica (Wien), 154, 1555-1561. https://doi.org/10.1007/s00701-012-1428-8
- 16. Yanagawa, Y. and Sakamoto, T. (2012) Results of Single Burr Hole Drainage for Acute Subdural Hematoma with Non-Reactive Pupil. Turkish Neurosurgery, 22, 196-199.
- 17. Leitgeb, J., Mauritz, W., Brazinova, A., Janciak, I., Majdan, M. and Wilbacher, I. (2012) Outcome after Severe Brain Trauma Due to Acute Subdural Hematoma. Journal of Neurosurgery, 117, 324-333. https://doi.org/10.3171/2012.4.JNS111448
- 18. Xu, H., Niu, C., Fu, X., Ding, W., Ling, S., Jiang, X. and Ji, Y. (2015) Early Cranioplasty vs. Late Cranioplasty for the Treatment of Cranial Defect: A Systematic Review. Clinical Neurology and Neurosurgery, 136, 33-40. https://doi.org/10.1016/j.clineuro.2015.05.031
- 19. Tasiou, A., Vagkopoulos, K., Georgiadis, I., Brotis, A.G., Gatos, H. and Fountas, K.N. (2014) Cranioplasty Optimal Timing in Cases of Decompressive Craniectomy after Severe Head Injury: A Systematic Literature Review. Interdisciplinary Neurosurgery, 1, 107-111. https://doi.org/10.1016/j.inat.2014.06.005
- 20. Clune, J.E., Mulliken, J.B., Glowacki, J., Arany, P.R., Kulungowski, A.M., Rogers, G.F. and Greene, A.K. (2011) Autologous Cranial Particulate Bone Graft: An Experimental Study of Onlay Cranioplasty. Journal of Craniofacial Surgery, 22, 319-323. https://doi.org/10.1097/SCS.0b013e3181f7e0e2




