Open Journal of Pathology
Vol. 2 No. 3 (2012) , Article ID: 21134 , 6 pages DOI:10.4236/ojpathology.2012.23017
Dedifferentiated Chondrosarcoma with a High-Grade Mesenchymal Component Mimicking a Gastrointestinal Stromal Tumor
![]()
Department of Orthopaedic Surgery, Graduate School of Medicine, Kobe University, Kobe, Japan.
Email: *akisue@med.kobe-u.ac.jp
Received March 4th, 2012; revised March 29th, 2012; accepted April 5th, 2012
Keywords: Dedifferentiated Chondrosarcoma; Gastrointestinal Stromal Tumor; c-kit; Histology; Immunohistochemistry
ABSTRACT
This report presents a dedifferentiated chondrosarcoma with a unique pathologic feature. A 63-year-old man was referred with pain and a soft tissue mass in the left groin. A plain radiograph showed a mineralization in the proximal femur with partially osteolytic foci and an abnormal shadow in the soft tissue. Magnetic resonance imaging scans showed an inhomogeneous lesion with intermediate to partially low signal intensity on T1-weighted image and intermediate to high signal intensity on T2-weighted image. Microscopically, the tumor in the femur is a low-grade chondrosarcoma and the component of soft tissue was a high-grade sarcomatous lesion with an epithelial arrangement of tumor cells. A diffuse immunoreactivity to both vimentin and c-kit (CD117) antibodies was detected in the high-grade component. A dedifferentiated component is similar to those of gastrointestinal stromal tumor (GIST). This is the first case of dedifferentiated chondrosarcoma with a high-grade component mimicking a GIST.
1. Introduction
Dahlin and Beabout first defined dedifferentiated chondrosarcoma in 1971 as a variant of chondrosarcoma that consisted of a benign or low-grade malignant cartilagenous component with a high-grade noncatilagious sarcomatous component [1]. Dahlin and Beabout noted that the high-grade components in dedifferentiated chondrosarcomas showed histologic features resembling fibrosarcoma or osteogenic sarcoma. After the first description of dedifferentiated chondrosarcoma, previous studies have shown that the high-grade component typically comprised of malignant fibrous histiocytoma, osteosarcoma, and fibrosarcoma [1-6], although several reports also have presented histologically unique dedifferentiated chondrosarcomas with high-grade areas mimicking rhabdomyosarcoma [7-9], leiomyosarcoma [10-12], and giant cell tumor [13-15]. To the authors’ knowledge, however, dedifferentiated chondrosarcoma with a high-grade component mimicking a gastrointestinal stromal tumor (GIST) has not been described in the English language literature. This report presents clinico-pathologic, radiographic, and immunohistochemical features of an unusual dedifferentiated chondrosarcoma with a gastrointestinal stromal tumor-like component.
2. Case Report
A 63-year-old man was referred with a soft tissue mass in the left groin accompanying with severe pain for 3 months. The pain gradually increased and eventually disturbed walking. The patient had a history of intertrochanteric fracture in his left femur at 14 years of age, which was treated with casting for 2 months. The patient complained of discomfort and pain in the anterior chest one month before the presentation to the hospital and the diagnosis was angina pectoris. On physical examination, an elastic hard mass with a rough surface was palpated in the medial aspect of the left groin, measuring approximately 15 cm in the greatest diameter. The mass strongly adhered to the femur, but not to the skin. The patient felt tenderness in the mass. There was mild swelling around the soft tissue mass without local heat or redness. The range of motion in the left hip was slightly restricted. The laboratory tests showed marked increases of white blood cell count (10.700/mm3), c-reactive protein (7.6 mg/dl), erythrosedimentation rate (98 mm/h) at the presentation to the hospital. Alkaliphosphatase (307 U/ml) and serum calcium (10.0 mg/dl) concentrations also slightly increased. An anteroposterior plain radiograph showed a mineralization in the proximal femur with partially osteolytic foci and an abnormal shadow in the soft tissue medially adjacent to the femur (Figure 1). Computed tomography (CT) scans showed a high-density area in the proximal part of the femur, which may be indicative of mineralization in the medulla (Figure 2(a)). Multiplanar reconstruction images of CT showed mineralizetion in the medulla and endosteal scalloping (Figure 2(b)). Post-contrast CT scans showed an inhomogeneous increase of density in both bone and soft tissue lesions. Magnetic resonance imaging (MRI) scans showed an inhomogeneous lesion with intermediate to partially low signal intensity on T1-weighted image (Figure 3(a)). T2- weighted images showed a distinct pattern of signal intensity in medulla of proximal femur and soft tissue lesion adjacent to the femur. On T2-weighted images, signal intensity in the medulla of proximal femur was low (boid) to partially very high, whereas soft tissue lesion adjacent to the femur showed intermediate to high signal intensity (Figure 3(b)). A gadolinium-enhanced T1- weighted image showed an inhomogeneous increase of signal intensity in both the femur and the soft tissue lesion (Figure 3(c)). A 99mTc-methylene diphosphonate scintigraph showed a diffuse uptake in the proximal femur on the left, but no accumulation of isotope was detected in the adjacent soft tissue (Figure 4). Systemic investigations for distant metastases were conducted using CT and MRI. CT scans for the thorax revealed multiple mass lesions indicative of metastases in the bilateral lung. No evidence of tumor in the gastrointestinal tract was detected under endoscopic examination.
The patient underwent an open biopsy for both bone and soft tissue components of the tumor. At biopsy, the soft tissue component showed a yellowish brown mass with a thin capsule-like tissue and the tumor in the femoral medulla consisted of ash-white friable tissue. Microscopically, the specimen from the femoral medulla consisted of cartilagious tissue, bone, and fibrous tissue (Figures 5(a) and (b)). Cartilagious tumor cells showed lowgrade nuclear atypia without multinucleated cells. Calcification was detected around the cartilaginous tissue. There was no osteoid in the specimen from the femoral medulla. The specimen from the femoral medulla was diagnosed as a low-grade chondrosarcoma (grade I) based on the criteria previously described [16]. The specimen from soft tissue mass consisted of pleomorphic sarcomatous cells and collagen fibers (Figures 5(c) and (d)). The tumor cells arranged in an epithelial growth pattern which is similar to the gastrointestinal stromal tumor. The nuclei of the tumor cells showed atypia with condensation of the vesicular chromatin and a prominent nucleolus. Mitoses also could be detected. The cytoplasm of the tumor cells was weakly eosinophilic and vesicular. The specimen stained with Masson trichrome showed

Figure 1. A plain radiograph showing a mineralization in the proximal femur with partially osteolysis in the left femoral neck.
 (a)
(a) (b)
(b)
Figure 2. A computed tomography scan showing a highdensity area in the medulla of femoral neck (a); A multiplanar reconstruaction image of CT showing mineralization in the medulla and endosteal scalloping (b).
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. Magnetic resonance imaging (MRI) scans showing intermediate to partially low signal intensity on T1-weighted image (a); And low to partially very high in the proximal femur and intermediate to high signal intensity in the soft tissue lesion on T2-weighted image (b); A gadolinium-enhanced T1-weighted image showing an increase of signal intensity in both the femur and the soft tissue lesions (c).

Figure 4. A 99mTc-methylene diphosphonate scintigraph showing a diffuse uptake in the left proximal femur.
cleaved collagen fibers, the amount of which varied form area to area (Figure 6). An alveolar growth pattern was not detected in the Gitter stained sections. There were no necrotic or hemorrhagic foci in the specimen from highgrade area.
Immunohistochemically, the tumor cells in the soft tissue component showed diffusely positive immunoreactivity for vimentin and c-kit (CD117) antibodies (Figures 7(a) and (b)). Weak immunoreactivity for bcl-2 protein and epithelial membrane antigen (EMA) antibodies was detected in low percentage of soft tissue component. The specimen from high-grade component did not show any immunoreactivity for desmin, alphasmooth muscle actin, S-100 protein, cytokeratin, CD34 antigen antibodies, HHF35, and HMB45.
The patient underwent an irradiation (45 Gy) for the tumor in the groin and a systemic chemotherapy (ifosfamide 8 g/body), but he died 3 months after presentation to the authors’ hospital.
3. Discussion
Dedifferentiated chondrosarcoma was first described by Dahlin and Beabout in 1971 as a variant of chondrosarcoma that consisted of a benign or low-grade malignant cartilaginous component with a high-grade noncatilagious sarcomatous component [1]. Approximately 10% - 15% of chondrosarcomas are diagnosed as dedifferentiated chondrosarcoma [3,4,16]. The high-grade noncatilagious component typically showed pathologic features resembling malignant fibrous histiocytoma (MFH), conventional osteosarcoma, and fibrosarcoma [2-4,6], although previous reports also have presented histologycally unique dedifferentiated chondrosarcomas with highgrade areas mimicking rhabdomyosarcoma [7-9], leiomyosarcoma [10-12], giant cell tumor [13-15], and telangiectatic osteosarcoma [5]. Microscopically, the specimen from the femoral medulla consisted of cartilagious tissue, bone, and fibrous tissue. The catilagious component in the femoral medulla, showing low-grade nuclear atypia without multinucleated cells, was diagnosed as a low-grade chondrosarcoma (garde I). The specimen from soft tissue mass, showing pleomorphic sarcomatous cells with mitoses, was diagnosed as high-grade sarcomatous component. Based on these microscopic findings, we diagnosed the current case as dedifferentiated chondrosarcoma. However, morphologic characteristics of highgrade component were not consistent with those of MFH,
 (a)
(a) (b)
(b)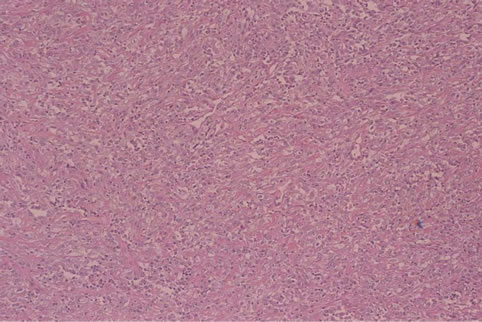 (c)
(c) (d)
(d)
Figure 5. The specimen from the femoral medulla consisting of cartilagious tissue, bone, and fibrous tissue ((a) low magnification, haematoxylin and eosin). Cartilagious tumor cells showing low-grade nuclear atypia without multinucleated cells ((b) high magnification). The specimen from soft tissue mass consisting of pleomorphic sarcomatous cells and collagen fibers ((c) low magnification). The tumor cells arrange in an epithelial growth pattern which is similar to the gastrointestinal stromal tumor ((d) high magnification).

Figure 6. Masson trichrome showing cleaved collagen fibers.
 (a)
(a) (b)
(b)
Figure 7. Immunohistochemical analysis showing diffusely positive immunoreactivity for vimentin (a) and c-kit (CD- 117) (b) antibodies in the tumor cells in high-grade mesenchymal component.
osteosarcoma, rhabdomyosarcoma, leiomyosarcoma, nor giant cell tumor.
Gastrointestinal stromal tumor (GIST) was first introduced as a mesenchymal tumor in the gastrointestinal tract by Mazur and Clark [17], although GIST had been thought to originate from smooth muscle cells in the gastrointestinal tract and had been called leiomyoma or leiomyosarcoma before the publication by Mazur and Clark. In 1998, it has been demonstrated that the cell origin of GIST might be the intestinal cells of Cajal or their precursors and that mutations of KIT gene in the GIST cells might initiate the tumorigenesis [18,19]. Extragastrointestinal stromal tumor (EGIST) was also recognized as a GIST arising from omentum, mesentery, or retroperitoneum and not connecting to gut wall or serosal surface of the viscera. The pathological findings of GIST and EGIST are comparable, which consist of epithelioid and spindle cells. Usually either epithelioid or spindle cells are predominant in the specimens of GIST, but sometimes equal distribution of both epithelioid and spindle cells is found. Immunochistochemical findings of GIST are characteristic and definitive for diagnosis. Almost all GIST show a positive immunorectitivity to KIT (CD117). About other antigens, CD34 is positive in 50% - 75%, smooth muscle actin (SMA) in less than 25%, and desmin or keratin in less than 5%. In the current case, high-grade component showed an epithelial growth pattern which is similar to the gastrointestinal stromal tumor. In terms of immunochistochemistry, the tumor cells in high-grade component showed diffusely positive immunoreactivity for vimentin and c-kit (CD117) antibodies, whereas negative immunoreactivity for desmin, alphasmooth muscle actin, S-100 protein, cytokeratin, CD34 antigen antibodies, HHF35, and HMB45. Based on these pathological and immunochemical findings, we diagnosed dedifferentiated chondrosarcoma with GIST-like features.
Radiologic features in the current case are comparable with previous studies [10,20,21]. Previous study have demonstrated that 77% of radiographs and 86% of CT scans for dedifferentiated chondrosarcomas showed intraossous calcification and that endosteal scalloping was detected in 67% of radiographs and 90% of CT scans. In the current case, plain Radiographs and CT scans showed calcification in the medulla of femur and endosteal scalloping of cortex, suggesting cartilaginous tumor with invasive behavior. In the previous studies, MR imaging have shown unique imaging features of dedifferentiated chondrosarcomas [10,20,21]. MacSweeney et al. have proposed three MRI patterns in dedifferentiated chondrosarcomas: type1, two distinct signals with hyper- intense chondral lesion and reduced signal intensity of dedifferentiated component; type 2, mainly reduced signal intensity with partial signal void corresponding to matrix mineralization; type 3, a heteroginous signal intensity without evidence of underlying chondral component [20]. It have been also reported that tumor bimorphism was detected 35%, 48%, and 33% of dedifferentiated chondrosarcomas in radiographs, CT scans, and MR images respectively [10]. MR images in the current case showed bimorphism in the medulla of femur and soft tissue component adjacent to the femur, which may be classified as type 1 based on the criteria by MacSweeney et al. Therefore, radiologic evaluation before biopsy was helpful to diagnose the current case as dedifferentiated chondrosarcomas and to plan a biopsy to get specimens of two distinct components. Michell et al. also have suggested that precise diagnosis before treatment may affect the prognosis of patients with dedifferentiated chondrosarcomas [22].
Dedifferentiated chondrosarcoma has a poor prognosis. The 5-year survival rate ranges form 10.5% - 29% [2-4,22-24]. Previous studies have shown prognostic factors for dedifferentiated chondrosarcoma. Michell et al. have proposed a benefit of chemotherapy for dedifferentiated chondrosarcoma [22], whereas Dickey et al. has declined the effect of chemotherapy on prognosis [3]. Grimer et al. conducted a multicenter study in Europe and analyzed 337 patients. It has been demonstrated that poor prognostic factors for over-all survival were metastasis at diagnosis, pathologic fracture at diagnosis, a pelvic location, age, and surgical margin [23]. The histological subtype, tumor size, and the use of chemotherapy did not correlate with prognosis [23]. The role of chemotherapy for dedifferentiated chondrosarcoma is still controversial. The current case underwent chemotherapy (ifosfamide) and a palliative irradiation, but only palliative effect could be detected without regression of the tumor. The dedifferentiated component mimicking a gastrointestinal stromal tumor may also be resistant to chemotherapy and radiotherapy, which result in poor prognosis. Further new treatment strategy including novel chemotherapy for dedifferentiated chondrosarcoma should be developed.
REFERENCES
- D. C. Dahlin and J. W. Beabout, “Dedifferentiation of Low-Grade Chondrosarcomas,” Cancer, Vol. 28, No. 2, 1971, pp. 461-466. doi:10.1002/1097-0142(197108)28:2<461::AID-CNCR2820280227>3.0.CO;2-U
- F. J. Frassica, K. K. Unni, J. W. Beabout and F. H. Sim, “Dedifferentiated Chondrosarcoma. A Report of the Clinicopathological Features and Treatment of SeventyEight Cases,” Journal of Bone & Joint Surgery, Vol. 68, No. 8, 1986, pp. 1197-1205.
- I. D. Dickey, P. S. Rose, B. Fuchs, L. E. Wold, S. H. Okuno, F. H. Sim and S. P. Scully, “Dedifferentiated Chondrosarcoma: The Role of Chemotherapy with Updated Outcomes,” Journal of Bone & Joint Surgery, Vol. 86, No. 11, 2004, pp. 2412-2418.
- J. Bruns, W. Fiedler, M. Werner and G. Delling, “Dedifferentiated Chondrosarcoma—A Fatal Disease,” Journal of Cancer Research and Clinical Oncology, Vol. 131, No. 6, 2005, pp. 333-339. doi:10.1007/s00432-004-0648-6
- K. Okada, T. Hasegawa, U. Tateishi, M. Endo and E. Itoi, “Dedifferentiated Chondrosarcoma with Telangiectatic Osteosarcoma-Like Features,” Journal of Clinical Pathology, Vol. 59, No. 11, 2006, pp. 1200-1202. doi:10.1136/jcp.2005.029629
- J. Sopta, A. Dordević, G. Tulić and V. Mijucić, “Dedifferentiated Chondrosarcoma: Our Clinico-Pathological Experience and Dilemmas in 25 Cases,” Journal of Cancer Research and Clinical Oncology, Vol. 134, No. 2, 2008, pp. 147-152. doi:10.1007/s00432-007-0262-5
- R. N. Astorino and H. Tesluk, “Dedifferentiated Chondrosarcoma with a Rhabdomyosarcomatous Component,” Human Pathology, Vol. 16, No. 3, 1985, pp. 318-320. doi:10.1016/S0046-8177(85)80022-8
- J. D. Reith, T. W. Bauer, D. F. Fischler, M. J. Joyce and K. E. Marks, “Dedifferentiated Chondrosarcoma with Rhabdomyosarcomatous Differentiation,” American Journal of Surgical Pathology, Vol. 20, No. 3, 1996, pp. 293-298. doi:10.1097/00000478-199603000-00005
- P. L. Munk, D. G. Connell and N. F. Quenville, “Dedifferentiated Chondrosarcoma of Bone with Leiomyosarcomatous Mesenchymal Component: A Case Report,” Canadian Association of Radiologists Journal, Vol. 39, No. 3, 1988, pp. 218-220.
- L. A. Littrell, D. E. Wenger, L. E. Wold, F. Bertoni, K. K. Unni, L. M. White, R. Kandel and M. Sundaram, “Radiographic, CT, and MR Imaging Features of Dedifferentiated Chondrosarcomas: A Retrospective Review of 174 de Novo Cases,” Radiographics, Vol. 24, No. 5, 2004, pp. 1397-1409. doi:10.1148/rg.245045009
- T. Akahane, T. Shimizu, K. Isobe, Y. Yoshimura and H. Kato, “Dedifferentiated Chondrosarcoma Arising in a Solitary Osteochondroma with Leiomyosarcomatous Component: A Case Report,” Archives of Orthopaedic and Trauma Surgery, Vol. 128, No. 9, 2008, pp. 951-953. doi:10.1007/s00402-008-0567-0
- H. A. Sissons, J. A. Matlen and M. M. Lewis, “Dedifferentiated Chondrosarcoma. Report of an Unusual Case,” Journal of Bone & Joint Surgery, Vol. 73, No. 2, 1991, pp. 294-300.
- T. Ishida, H. D. Dorfman and E. T. Habermann, “Dedifferentiated Chondrosarcoma of Humerus with Giant Cell Tumor-Like Features,” Skeletal Radiology, Vol. 24, No. 1, 1995, pp. 76-80. doi:10.1007/BF02425959
- E. G. Estrada, A. G. Ayala, V. Lewis and B. Czerniak, “Dedifferentiated Chondrosarcoma with a Noncartilaginous Component Mimicking a Conventional Giant Cell Tumor of Bone,” Annals of Diagnostic Pathology, Vol. 6, No. 3, 2002, pp. 159-163. doi:10.1053/adpa.2002.33905
- R. Arora, A. Sharma and A. K. Dinda, “Dedifferentiated Chondrosarcoma of the Femur Mimicking a Conventional Giant Cell Tumor: A Diagnostic Pitfall,” Indian Journal of Pathology and Microbiology, Vol. 51, No. 4, 2008, pp. 561-562. doi:10.4103/0377-4929.43763
- M. E. Pring, K. L. Weber, K. K. Unni and F. H. Sim, “Chondrosarcoma of the Pelvis. A Review of Sixty-Four Cases,” Journal of Bone & Joint Surgery, Vol. 83, No. 11, 2001, pp. 1630-1642.
- M. T. Mazur and H. B. Clark, “Gastric Stromal Tumors. Reappraisal of Histogenesis,” American Journal of Surgical Pathology, Vol. 7, No. 6, 1983, pp. 507-519. doi:10.1097/00000478-198309000-00001
- S. Hirota, K. Isozaki, Y. Moriyama, K. Hashimoto, T. Nishida, S. Ishiguro, K. Kawano, M. Hanada, A. Kurata, M. Takeda, G. M. Tunio, Y. Matsuzawa, Y. Kanakura, Y. Shinomura and Y. Kitamura, “Gain-of-Function Mutations of c-kit in Human Gastrointestinal Stromal Tumors,” Science, Vol. 279, No. 5350, 1998, pp. 577-580. doi:10.1126/science.279.5350.577
- L. G. Kindblom, H. E. Remotti, F. Aldenborg and J. M. Meis-Kindblom, “Gastrointestinal Pacemaker Cell Tumor (GIPACT): Gastrointestinal Stromal Tumors Show Phenotypic Characteristics of the Interstitial Cells of Cajal,” American Journal of Pathology, Vol. 152, No. 5, 1998, pp. 1259-1269.
- S. Eustace, N. Baker, H. Lan, A. Wadhwani and D. Dorfman, “MR Imaging of Dedifferentiated Chondrosarcoma,” Clinical Imaging, Vol. 21, No. 3, 1997, pp. 170-174. doi:10.1016/S0899-7071(96)00016-2
- F. MacSweeney, A. Darby and A. Saifuddin, “Dedifferentiated Chondrosarcoma of the Appendicular Skeleton: MRI-Pathological Correlation,” Skeletal Radiology, Vol. 32 No. 12, 2003, pp. 671-678. doi:10.1007/s00256-003-0706-1
- A. D. Mitchell, K. Ayoub, D. C. Mangham, R. J. Grimer, S. R. Carter and R. M. Tillman, “Experience in the Treatment of Dedifferentiated Chondrosarcoma,” Journal of Bone & Joint Surgery, British Volume, Vol. 82, No. 1, 2000, pp. 55-61. doi:10.1302/0301-620X.82B1.9020
- R. J. Grimer, G. Gosheger, A. Taminiau, D. Biau, Z. Matejovsky, Y. Kollender, M. San-Julian, F. Gherlinzoni and C. Ferrari, “Dedifferentiated Chondrosarcoma: Prognostic Factors and Outcome from a European Group,” European Journal of Cancer, Vol. 43, No. 14, 2007, pp. 2060-2065. doi:10.1016/j.ejca.2007.06.016
- E. L. Staals, P. Bacchini, M. Mercuri and F. Bertoni, “Dedifferentiated Chondrosarcomas Arising in Preexisting Osteochondromas,” Journal of Bone & Joint Surgery, Vol. 89, No. 5, 2007, pp. 987-993. doi:10.2106/JBJS.F.00288
NOTES
*Corresponding author.

