Natural Resources
Vol.4 No.1(2013), Article ID:29168,4 pages DOI:10.4236/nr.2013.41007
The Effect of NaHCO3 as Catalyst via Electrolysis
![]()
Department of Science Engineering, Faculty of Sciences and Technology, University of Malaysia Terengganu, Terengganu, Malaysia.
Email: *zam@umt.edu.my
Received March 27th, 2012; revised July 3rd, 2012; accepted August 5th, 2012
Keywords: Hydrogen; Water Electrolysis; Stainless Steels; NaHCO3
ABSTRACT
Renewable energy is a kind of energy that comes from natural sources like water, sunlight, wind and so on. Water electrolysis is currently the most dominant technology used for hydrogen production from renewable sources because of high energy conversion efficiency. In this present work, the effect of NaHCO3 via electrolysis was studied. Stainless steel is chosen to be as the electrode and different concentrations of NaHCO3 are used as alkaline solutions in electrolysis system. The rates of hydrogen gas produced using different concentration of NaHCO3 and pH value of every sample were measured. The experimental results that the performance of water electrolysis was highly affected by NaHCO3, the rate of hydrogen gas showed that 0.4 M of NaHCO3 and 65 ml/min at pH 8.2 are the best amount.
1. Introduction
Nowadays, energy has always been the primary focus of mankind and it continues to drive the economy through a series of technological advances. The energy-based industrial and scientific revolution, places a demand on researchers and industries to produce sustainable energy technologies. Hydrogen is considered as an idea for future energy carrier. It can be produced from renewable energy [1]. One of the most promising methods for hydrogen production is water electrolysis using various energy sources which can be obtained from solar, geothermal, hydroelectric and nuclear [2]. A basic water electrolysis unit consists of an anode, a cathode, power supply, and an electrolyte [3] and does not cause air pollution [4]. Nowadays most of the hydrogen in this world is from fossil fuels. Conversion of chemical energy that stored in fossil fuels or in nuclear processes has been the major contributor for the world’s energy demand. However the combustion of fossil fuels spews out toxic substances like COx, NOx, SOx etc., into the air and causes air pollution. Hydrogen plays a key role as an energy storage media and it can be generated by various techniques. The production of Hydrogen via water electrolysis is still considered to be the low cost alternative way, if energy efficient techniques are established. The main advantage of electrolysis is very pure hydrogen gas can be produced, unlike other processes. Water electrolysis is often considered as the preferred method of hydrogen production as it is the only process that need not rely on fossil fuels. It also has the high product purity, and is feasible on small and large scales [5].
Currently, the studies of hydrogen via electrolysis that considering the effect of current distance between the electrode, and the temperature on efficiency of alkaline water electrolysis at a particular concentration of the solution is popular among the researchers. In previous research, catalysts are used in electrolysis as electrolyte with water or without water to produce more hydrogen gas. Catalysts that used currently are potassium hydroxide [4,5], methanol [6], sulphuric acid [1], ammonia [7] and so on. For this research, NaHCO3 are used as catalyst via electrolysis and no one researcher report about NaHCO3.
The main objective in this study is to see the performance electrolysis system using NaHCO3 as catalyst and the rate of hydrogen produced using NaHCO3 as electrolyte. All these experiments are done in under room temperature.
2. Methodology
2.1. Sample Preparation
In this research, electrolyte solutions were prepared using NaOH and NaHCO3 at different concentration and deionized water. The concentrations of the samples were listed in Table 1. The electrodes used were stainless steel electrode [8]. Stainless steel 316 with geometrical area 11 cm2 were used [9]. The electrodes were polished
Table 1. The concentration of the NaHCO3 and NaOH respectively.

before used.
2.2. Weight Loss Measurement
The weight loss measurement is a classical way to determine the corrosion effect of electrode after electrolysis occurred. The initial weight of electrode (w0) and the final weight of electrode after electrolysis process (w1) were weighed.
 (1)
(1)
2.3. Experiment Apparatus
The container with height 60 cm × 30 cm × 30 cm was built, to measure the hydrogen gas. After that, a beaker with volume of 1000 ml was placed upside down inside the container. Then, water was poured into the container until the 1000 ml beaker is filled with water. Before that, a tube was plastered on the beaker and attached to the electrolysis device. 12 V of power supply was used during the electrolysis process. Gas that was produced during the electrolysis process was delivered through the tube into the beaker in the container. Throughout that process, the water level inside the beaker was reduced. Time reading adopt for gas production was taken when the water level inside the beaker was from 0 ml to 200 ml, 400 ml, 600 ml, 800 ml, and 1000 ml. When the level of water achieves the point of 1000 ml, electrolysis process halted and the samples undergo further electrolysis process.
In order to verify the amount gas produced by stainless steel electrode, parameters as follows are controlled such as the gap between two electrodes, the size of electrode, various voltages input for alkaline water electrolysis model and vertically the setting of electrode. The experimental water electrolysis test model was shown in Figure 1 and the component used in test model is listed in Table 2. Five test model based on NaHCO3 (0.2 M - 1.0 M) was produced. The components that listed in Table 1 were set up before experiment was started. The electrode in vertical position in the electrolysis container with the electrolyte and the experiment was started function. The voltage, current, weight of electrode, pH solution and the water mass before and after the test are measured and the rates of production are calculated in 1 hour for different concentration electrolyte. This experiment was repeated using NaOH and water without catalyst.

Figure 1. The alkaline water electrolysis test model (Nagai et al., 2003).
Table 2. Component used in alkaline water electrolysis test model experiment.

2.4. Gas Hydrogen Measurement
During the process of electrolysis, gas that released from the H2 tube was recorded. From that result, the rate of gas produced was calculated. The rate of gas produced was calculated by following the equation:
The rate of gas produced  (2)
(2)
where  are different of volume of water and
are different of volume of water and  are different time.
are different time.
2.5. pH Measurement
pH meter was used to measure pH for each sample. In this pH’s meter, there are several probe and buffer solutions which are used in this study. When calibration was carried out, pH value of the samples was measured in different concentration. pH value was calculated by using the following equation:
 (3)
(3)
 (4)
(4)
3. Results and Discussion
3.1. Weight Loss Measurement
To determine the corrosion effect of electrode after electrolysis process, the loss weight measurement is needed
[10]. The result of weight loss measurement was exposed in Figure 2. Based on Figure 2, it shows that the weight loss of electrode increases, with respect to the increasing of concentration. The weight loss of electrodes in the samples of NaHCO3, and NaOH is approximately similar to the weight loss of electrodes in the water sample under the process of electrolysis. This result is gotten using Equation (1).
The difference in the weight loss had shown when the concentrations of the electrolyte increase, the weight loss also increases because the solution becomes more alkaline and effect electrode. Besides that, the weight loss of electrodes using NaHCO3 is lower than NaOH and we can say that the two catalysts roughly have the same value of electrodes’ weight loss with water which is without catalyst. It has been found that in some cases the corrosion was not removed entirely while in other cases some of the base metal was removed along with corrosion [11].
3.2. The Rate of Hydrogen Produced
Figure 3 shows the rate of hydrogen that was calculated by using Equation (2). The graph in Figure 2 explains that the rate of hydrogen produced using NaHCO3 was higher than NaOH. At the concentration of 0.4 M, 65 ml/min by NaHCO3 and 63 ml/min by NaOH of hydrogen gas was produced respectively.
In this principle, the increasing amount of catalyst can increase the reaction rate of producing gas but it has optimum point to produce higher producing gas. It shows that the concentration of the electrolyte is important to produce hydrogen. Besides that, according to [12], it can be seen that all catalyst were used and showed some reaction compared with the standard electrolyte (water). In addition, from this research we can know that NaHCO3 is a good catalyst compared with NaOH. NaHCO3 shows an optimum level of concentration as a catalyst to produce hydrogen gas.
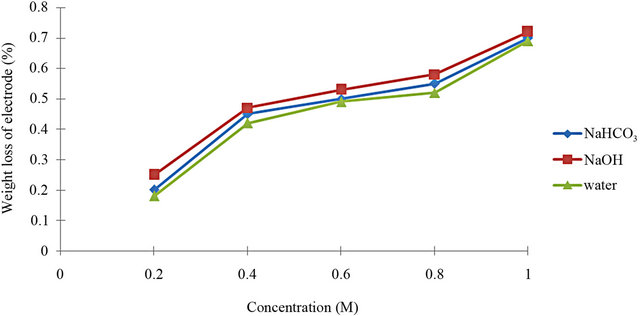
Figure 2. The weight loss verses sample.

Figure 3. The rate of hydrogen produced using different concentration of NaHCO3, NaOH and water. Applied voltage for electrolysis was 12 V.
Table 3. The pH value in different concentration.

Besides that, the performances of electrolysis via the rate of hydrogen produced are highly affected by catalyst. So, electrolysis can produce more hydrogen using NaHCO3 catalyst.
3.3. pH Measurement
The stainless steel electrodes exhibited pronounced using weight loss method in Figure 2, hydrogen producing rate in Figure 3 and also pH changes of the different concentration (Table 3) at all samples. The analysis of variance suggests that types of electrode materials and pH, as well as their interactions, have significant effects on the production rate. As we can see from Table 3, the different pH level can cause different type of gas to be produced. On the other hand, the weight loss of stainless steel, gas production and the pH value at 0.4 M of NaHCO3 and NaOH are good results. The results of this research show that stainless steel can be the good electrode to carry out water electrolysis process to produce hydrogen [13]. Therefore, NaHCO3 also can be used as electrolyte since it shows the efficient as catalyst to speed up the water electrolysis process.
4. Conclusion
From this research, it can be concluded that NaHCO3 can be used as catalyst to produce hydrogen gas and the performances of electrolysis affected by catalyst are good.
5. Acknowledgements
The first author would like to thank Universiti Malaysia Terengganu for the scholarship as well to Department of Science Engineering and Department of Physical Sciences for their help in this research.
REFERENCES
- D. Graf, N. Monnerie, M. Roeb, M. Schmitz and C. Sattler, “Economic Comparison of Solar Hydrogen Generation by Means of Thermochemical Cycles and Electrolysis,” International Journal of Hydrogen Energy, Vol. 33, No. 17, 2008, pp. 4511-4519. doi:10.1016/j.ijhydene.2008.05.086
- W. Hu, “Electrocatalytic Properties of New Electrocatalysts for Hydrogen Evolution in Alkaline Water Electrolysis,” International Journal of Hydrogen Energy, Vol. 25, No. 2, 2000, pp. 111-118. doi:10.1016/S0360-3199(99)00024-5
- K. Zeng and D. Zhang, “Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications,” Progress in Energy and Combustion Science, Vol. 36, No. 3, 2010, pp. 307-326. doi:10.1016/j.pecs.2009.11.002
- F. L. Petrik, Z. G. Godongwana and E. I. Iwuoha, “Plantinum Nanophase Electro Catalyst and Composite Electrodes for Hydrogen Production,” Journal of Power Sources, Vol. 185, No. 2, 2008, pp. 838-845. doi:10.1016/j.jpowsour.2008.06.091
- C. Koroneos, A. Dompros, G. Roumbas and N. Moussiopoulos, “Life Cycle Assessment of Hydrogen Fuel Production Processes,” International Journal of Hydrogen Energy, Vol. 29, No. 14, 2004, pp. 1443-1450. doi:10.1016/j.ijhydene.2004.01.016
- N. Nagaia, M. Takeuchia, T. Kimurab and T. Okaa, “Existence of Optimum Space between Electrodes on Hydrogen Production by Water Electrolysis,” International Journal of Hydrogen Energy, Vol. 28, No. 1, 2003, pp. 35-41. doi:10.1016/S0360-3199(02)00027-7
- T. Take, K. Tsurutani and M. Umedab, “Hydrogen Production by Methanol-Water Solution Electrolysis,” Journal of Power Sources, Vol. 164, No. 1, 2007, pp. 9-16. doi:10.1016/j.jpowsour.2006.10.011
- C. Zamfiresa and I. Dincer, “Ammonium as a Green Fuel and Hydrogen Source for Vehicular Application,” Fuel Processing Technology, Vol. 90, No. 5, 2009, pp. 729- 737. doi:10.1016/j.fuproc.2009.02.004
- C. P. Samaranayake and S. K. Sastry, “Electrode and pH Effects on Electrochemical Reactions during Ohmic Heating,” Journal of Electroanalytical Chemistry, 2005, pp. 125-135.
- J. M. Olivares-Ramírez, M. L. Campos-Cornelio and J. Uribe Godínez, “Studies on the Hydrogen Evolution Reaction on Different Stainless Steel,” International Journal of Hydrogen Energy, Vol. 32, No. 15, 2007, pp. 3170- 3173. doi:10.1016/j.ijhydene.2006.03.017
- R. O. Rihan and S. Nešić, “Erosion-Corrosion of Mild Steel in Hot Caustic. Part I: NaOH Solution,” Journal of Corrosion Science, Vol. 48, No. 9, 2006, pp. 2633-2659. doi:10.1016/j.corsci.2005.09.018
- D. L. Stojić, M. P. Marčeta, S. P. Sovilj and S. S. Miljanić, “Hydrogen Generation from Water ElectrolysisPossibilities of Energy Saving,” Journal of Power Sources, Vol. 118, No. 1, 2003, pp. 315-319. doi:10.1016/S0378-7753(03)00077-6
- L. Vracar and B. E. Conway, “Temperature Dependence of Electrocatalytic Behavior of Some Glassy Transition Metal Alloys for Cathodic Hydrogen Evolution in Water Electrolysis,” International Journal Hydrogen Energy, Vol. 15, No. 10, 1990, pp. 701-713. doi:10.1016/0360-3199(90)90001-F
NOTES
*Corresponding author.

