Open Journal of Ecology
Vol.06 No.09(2016), Article ID:69933,11 pages
10.4236/oje.2016.69055
Segregated Distribution of Liriope tetraphylla, Aglaura hemistoma and Nausithoe punctata (Cnidaria) in the Southern Gulf of Mexico
César Flores-Coto1*, Alejandro Puente-Tapia1, Laura Sanvicente Añorve1, Mariana Fernández-Alamo2
1Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Ciudad Universitaria, México D.F., Mexico
2Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad Universitaria, México D.F., Mexico

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 13 July 2016; accepted 18 August 2016; published 22 August 2016
ABSTRACT
In order to study the segregated distribution of the three most abundant jellyfish species in the southern Gulf of Mexico, a total of 85 stations were sampled during an oceanographic cruise from 19 May to 18 June 2006. Trawling took place from surface to a maximum depth of 200 m, using a Bongo net with a 61 cm mouth diameter and 333 and 500 µm mesh sizes. Temperature and salinity were recorded. Samples were preserved in 4% formalin, neutralized with sodium borate, and changed to 70% ethylic alcohol after 24 hours for conservation. The jellyfish data were standardized to 100 m3 of filtered water. A total of 10,610 jellyfish were collected from the 333 µm mesh size net, of which eight species represented 88.49% of the total density: Aglaura hemistoma, Liriope tetraphylla, Nausithoe punctata, Clytia hemisphaerica, Persa incolorata, Obelia spp., Clytia folleata and Eutima gracilis. The former three species are the subject of this study. The results obtained indicate that the high density areas of these three species have a segregated distribution. Segregation values (White’s index) recorded between pairs of specie were very high: L. tetraphylla - A. hemistoma, 0.88; L. tetraphylla - N. punctata, 0.86 and A. hemistoma - N. punctata, 0.84. The spatial distribution of the high density areas of these species fits well with the three hydrodynamically different areas: A. hemistoma in Campeche Bank, L. tetraphylla on the Campeche and Tabasco shelves and N. punctata in Campeche Bay. This spatial distribution pattern corresponds to their main habitat and reproductive habits of the species, as well as the influence of the hydrodynamics that dominate each area.
Keywords:
Cnidarians Segregated Distribution, Southern Gulf Mexico

1. Introduction
Jellyfish are planktonic invertebrates that are distributed mainly in marine and coastal environments [1] [2] . A general estimation is that there are approximately 40,000 marine and freshwater species.
They are a successful group as a result of their abundance in a wide variety of aquatic environments. Their geographical distribution ranges from polar to tropical areas and in the water column they live from the surface to depths of 2000 m [3] [4] .
Ecologically, their relevance lies in the part they play in trophic chains as predators, mainly at the top levels of the chains, with a diet that includes from diatoms and dinoflagellates to fish eggs and larvae, and practically all zooplanktonic groups [5] - [9] .
Jellyfish distribution varies depending on the biology of each species and on the prevailing oceanographic conditions. Among the most important biotic parameters is the availability of food, and among the abiotic ones are the fronts, haloclines, thermoclines, light, currents and turbulence [10] [11] , apart from physical-chemical- biological interactions [12] - [15] .
An important aspect of these organisms is that they tend to form blooms which, more than being beneficial to some groups of predators, generally constitute a bother, stopping up water inputs at nuclear plants or invading tourism areas.
The accumulation of jellyfish around physical discontinuities is a recurrent theme, particularly in fronts, upwellings and pycnoclines. Examples of local aggregations are common in the literature [14] . In some cases, such as that of Stomolophus meleagris, a commercially exploited species commonly called “cannonball jellyfish”, the great aggregations make them easy to collect [16] .
Much has been studied regarding jellyfish blooms phenomenon [14] , but there are not studies on spatial segregation.
Large proliferations of some species may occur during a certain period of the year, or in a certain area, in such a way that two or more species may be present with a high abundance and at the same time, but in different areas. It is this separation of species that may be called segregation. However, other jellyfish species, with a much lower abundance in comparison with the abundant species, generally appear together.
This is not an exclusive phenomenon of jellyfish, as it has also been recorded for appendicularians [17] - [22] .
Several studies have been carried out, with different sampling areas, periods and sampling intensity, on the seasonal variation, distribution and abundance of jellyfish throughout the Gulf of Mexico and Mexican Caribbean [23] - [32] . None of these studies, however, emphasized the marked differences that existed in the distribution of some species, particularly the more abundant and frequent ones. Thus, the purpose of this study was to determine the segregated distribution of the three most abundant species in the southern Gulf of Mexico, and to try to understand the causes of such segregation.
2. Study Area
The study area is located within the coordinates 18˚ to 23˚N and 96˚ to 87˚W, off the coasts of and in the oceanic region of the states of Veracruz, Tabasco, Campeche and Yucatán (Figure 1).
Three different areas may be distinguished in the study area: the Campeche Bank, the Campeche Bay and the coastal area, that is, the continental shelf off the states of Campeche, Tabasco and southern Veracruz. Campeche Bank is an extensive calcareous shelf [32] that corresponds to the continental shelf of the Yucatán peninsula, with maximum depths of 100 m, while Campeche Bay is the oceanic region of the southern Gulf of Mexico (Figure 1).
The dynamics in the study area are characterized by different meso-scale oceanographic events that determine differences among the areas. In Campeche Bank, the main event is a branch of the Yucatán current that moves from east to west [33] [34] . In Campeche Bay, the characteristic event is a semi-permanent cyclonic gyre that moves over the narrow Veracruz shelf and the outermost part of the Campeche and Yucatán shelves [35] [36] .
Figure 1. Study area and location of the sampling stations.
The main hydrographic event on the shelf off the coasts of Campeche, Tabasco and southern Veracruz is the great inflow of freshwater [37] . Zavala-Hidalgo and Fernández-Eguiarte [38] proposed a regionalization of the Gulf of Mexico based on physical processes, and those of the southern region closely correspond to those mentioned above.
3. Materials and Methods
Biological and hydrological data were obtained from 85 sampling stations during an oceanographic cruise on board the R/V “Justo Sierra” of the Universidad Nacional Autónoma de México, from 19 May to 18 June 2006.
Trawling for zooplancton took place at each station from the surface down to a maximum depth of 200 m where bathymetry allowed, using a Bongo type net with a 61 cm mouth diameter and 333 and 500 µm mesh sizes. Flowmeters were placed at the mouths of the nets. Temperature (˚C) and salinity were recorded at all stations with a General Oceanic Mark IV Neil Brown CTD.
Samples were preserved in 4% formalin, neutralized with Sodium Borate, and changed to 70% ethylic alcohol after 24 hours for conservation.
All jellyfish collected in the 333 µm mesh size net were quantified and identified to the lowest taxonomic level possible. The quantification of the specimens was standardized to 100 m3 of filtered water and expressed as org./100 m3.
The spatial segregation was analyzed by the D index [39] .
Equation (1) is
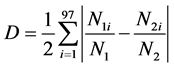
where
N1i = number of individuals of species 1 at station i,
N2i = number of individuals of species 2 at station i,
N1 = total number of individuals of species 1,
N2 = total number of individuals of species 2.
Spatial segregation values vary from 0 to 1, and indicate no segregation and perfect segregation respectively.
A Pearson correlation analysis was also applied between species density ((Ln(density + 1)) and temperature and salinity.
4. Results
4.1. Species
A total of 10,610 jellyfish were collected and identified, of which eight species represented 88.49% of the total density: Aglaura hemistoma, Liriope tetraphylla, Nausithoe punctata, Clytia hemisphaerica, Persa incolorata, Obelia spp., Clytia folleata and Eutima gracilis. The former three species are the subject of this study, with the greatest abundance recorded for the first, and decreasing thereafter for the following species (Table 1).
4.2. Temperature and Salinity
Temperature in the 30 m surface water layer varied from 23.4 to 29.1˚C. The higher values (>27˚C) were recorded both on the continental shelf and in the oceanic region adjacent to the states of Campeche, Tabasco and Veracruz, with an area of lower temperatures south of Veracruz, whereas the lower temperatures (<27˚C) were recorded on the Yucatán shelf (Figure 2(a)).
Salinity was relatively homogeneous, mostly with values above 36, except for some areas off the river mouths of Tabasco and southern Veracruz (Figure 2(b)). The higher values (>37) were recorded off Términos lagoon.
4.3. Abundance Distribution
The three species were collected practically throughout the study area. However, they presented different centers of high abundance. Aglaura hemistoma, the most abundant, was more concentrated in Campeche Bank and was particularly scarce in Campeche Bay, L. tetraphylla, the second in abundance and most widely distributed, mainly occupied the shelf off Campeche and Tabasco, and N. punctata was present in the deeper part of Campeche Bay and the most external area of the continental shelf from Veracruz to Tabasco (Figure 3).
The correlation analyses provided only three statistically significant values. One was for A. hemistoma, negative with temperature. One may clearly see, in the map with the distribution of temperature values, the lower temperatures in Campeche Bank where the high concentrations of this species were recorded (Table 2).
The other two values corresponded to L. tetraphylla and N. punctata, positive and negative respectively with salinity (Table 2), following that the centers of greater concentration of the first species were in high salinity areas on the Campeche shelf off Términos lagoon, while those of the second species were off the coast of Tabasco in a low salinity area.
The segregation indices among these species were very high (Table 3), indicating that there must be factors that affect the distribution of their centers of greater abundance, independently of the species’ own biology.
5. Discussion
5.1. Segregated Distribution
The three species studied here are the most abundant and frequent (Table 1) in the southern Gulf of Mexico, although seasonal variations may lead other species to occupy one of the first places [24] [26] [28] [29] [31] .
Table 1. Density (organisms 100 m−3) of the three species analyzed into the different areas in the southern Gulf of Mexico (May-June 2006) and total density and frequency (%).
Figure 2. Isopleths for (a) temperature and (b) salinity in the southern Gulf of Mexico (May-June 2006). Discontinuous line = 200 m isobath.
The results obtained in this study indicate that, notwithstanding the wide distribution of these three species in the southern Gulf of Mexico, their high density areas have a segregated distribution (Figure 3), as was confirmed by the high values obtained with the segregation index (Table 2).
Despite the fact that no studies have referred specifically to segregation among jellyfish, the phenomenon has already been recorded, and we take as an example the study of Buecher et al. [40] . These authors collected samples in the Mediterranean Sea for 27 years and observed that the species Pelagia noctiluca and L. tetraphylla never coincided, although their segregation was more temporal than spatial. Nearer our study area, Segura-Pu- ertas [24] observed a clear difference between the centers of greatest abundance of L. tetraphylla and A. hemistoma over two months of sampling in the Mexican Caribbean.
Figure 3. Density distribution of the three jellyfish species in the southern Gulf of Mexico (May-June 2006). Discontinuous line = 200 m isobath.
Table 2. Pearson correlation values and statistical probability between salinity and temperature, and species (N = 85).
Table 3. Segregation values (White’s index) recorded between pairs of species in the southern Gulf of Mexico (May-June 2006).
5.2. Hydrodynamics and Segregation
The spatial distribution of the high density centers of the species analyzed here fits well with the three hydrodynamically different areas: A. hemistoma in Campeche Bank, L. tetraphylla mainly on the Campeche shelve and N. punctata in Campeche Bay. This shows that, apart from the biological aspect that corresponds to their main habitat and reproductive habits, the distribution of these species is strongly affected by the hydrodynamics that dominate each area.
The cnidarians distribution pattern correlated with hydrographic pattern seems to be a common fact. According to Graham et al. [14] , jellyfish blooms are influenced in their magnitude and extent by the biology and behavior of the organisms, but also by the geographic setting and physical environment.
Aglaura hemistoma had its greatest density values in Campeche Bank where water circulation is dominated by the Yucatán current which, when it meets the slope of the continental shelf, causes an upwelling of deep water in the northern part of the Yucatán peninsula [41] [42] and generates a great primary productivity and, thus, an increased abundance in the following trophic levels up to the jellyfish. The high densities of A. hemistoma in these cold waters determined its negative correlation with temperature. High densities of this species were recorded by Segura-Puertas [24] also in the Campeche Bank in April and October (12,263 and 7924 organisms 100 m-3 respectively) and low densities < 125 org 100 m−3) en January and July.
In agreement with Colin et al. [43] A. hemistoma is an omnivorous jellyfish that feeds on microplankonic prey and often coincides with seasonal phytoplankton blooms.
Flores-Coto et al. [22] stated the possibility that the segregation of the filter feeding genera Oikopleura and Fritillaria (Larvacea) is associated with prey size. In the case of this study, however, another or other factors may be involved, as jellyfish are not filter feeders. For example, these same authors recorded a very low turbidity in Campeche Bank and a high turbidity in the area with large aggregations of L. tetraphylla.
The greatest densities of L. tetraphylla were recorded on the continental shelf off Campeche, and lesser extent in Tabasco and southern Veracruz shelves, an area where the main hydrographic event is the great inflow of freshwater. However, the Campeche shelf off Términos lagoon, where high densities of this species were recorded, is an area with very low freshwater runoff [36] , but with a considerable effect caused by the interchange of lagoon and coastal waters that characterizes it as a shallow, high temperature and high salinity area. High concentrations of this species have been recorded in association with estuarine fronts. Ueno and Mitsutani [44] and Loman-Ramos et al. [29] show the highest densities of L. tetraphylla also on the Campeche shelf.
Buecher et al. [39] analyzed 37 years of data and found that this species avoids low salinity areas, with about 50% of the population present in water with salinity values above 38.2. Liriope tetraphylla is a dominant carnivorous gelatinous zooplanktonic species, positively influenced by environmental conditions that favor increases in coastal planktonic production.
The distribution of N. punctata in Campeche Bay appears to be influenced by the cyclonic gyre that moves along the narrow shelf off Veracruz and in the most external region of the Tabasco, Campeche and Yucatán shelves [34] [35] . This distribution was negatively correlated with salinity, with the greater densities in the low salinity areas off the coasts of Tabasco and southern Veracruz, notwithstanding the oceanic nature of the species [45] .
The wide distribution of these three species indicates that they can live throughout a wide salinity and temperature range, although it is probable that their reproductive processes are conditioned or favored by some factor. Arai [46] indicated that aggregations are due to a combination of effects of physical forces and behavioral responses and that some species are found over broad ranges of temperature and salinity and despite there are many data correlating jellyfish abundance with the temperature and salinity of the water they live in, it has seldom been tested whether an environmental parameter directly affects the survival or growth of the jellyfish.
5.3. Recurrent Pattern
The distribution of these species seems to have a recurrent pattern as, at least the three previous studies carried out in the area, those of Valencia-Correia [25] , Segura-Puertas [24] and Loman-Ramos et al. [29] observed distributions that were very similar to those recorded in the present study. Loman-Ramos et al. [29] found that L. tetraphylla represented more than 78% of the fauna collected in Autumn, with the greatest densities on the Campeche shelf off Laguna de Términos, at depths low of 50 m, where temperature, salinity and zooplanktonic biomass values were greatest, in practically the same area and high salinity and temperature conditions we recorded in our study. Valencia-Correia [25] observed very similar results.
Segura-Puertas [24] obtained few data for N. punctata in the eastern part of the Yucatán shelf, and Valencia- Correia [25] recorded even less data in Campeche Bay. Loman-Ramos et al. [29] characterized it as an oceanic species, however presenting its greatest abundance on the narrow continental shelves of the states of Veracruz and Tabasco, particularly off the coasts of southern Veracruz, as was recorded in our study.
Regarding A. hemistoma, Segura-Puertas [24] and Valencia-Correia [25] recorded its greatest densities in the neritic waters of the Campeche Bank, as was recorded in our study but in contrast with the findings of Loman- Ramos et al. [29] .
6. Conclusions
It can be understood that the distribution of each species varies depending on oceanographic events and his own biology, which necessarily implies an appropriate availability of food for individual and population growth. It is necessary to carry out space-time studies as frequently as possible in a defined area to be able to relate the changes in populations with possible environmental agents that induce them. The sampling period was short in this case, however, it may be concluded that there is a clear spatial segregation in the abundance distribution of the three species, A. hemistoma, occupying the Campeche Bank, L. tetraphylla the Campeche and Tabasco shelf and N. punctata mainly in the deeper part of Campeche Bay and the most external area of the continental shelf from Veracruz to Tabasco.
On the other hand, the concordance of the centers of high abundance of these species with three different hydrodynamics areas, as well as the segregation of other groups coinciding with these same areas and finally similar distributions of the studied species recorded in previous works, allows us to consider that is most important the relationship between biotic and hydrological aspects that one or other separately, into the segregated distribution of these species.
Acknowledgements
The authors thank Faustino Zavala for the technical support provided. The samples for this study were collected during the PEMEX oceanographic cruise SGM 11.
Cite this paper
César Flores-Coto,Alejandro Puente-Tapia,Laura Sanvicente Añorve,Mariana Fernández-Alamo, (2016) Segregated Distribution of Liriope tetraphylla, Aglaura hemistoma and Nausithoe punctata (Cnidaria) in the Southern Gulf of Mexico. Open Journal of Ecology,06,568-578. doi: 10.4236/oje.2016.69055
References
- 1. Larson, R.J. (1986) Feeding and Growth of the Sea Nettle Chrysaora quinquecirrha (DeSor) in the Laboratory. Estuaries, 9, 376-379.
http://www.jstor.org/stable/1351418 http://dx.doi.org/10.2307/1351418 - 2. Suchman, C.L. and Sullivan, B.K. (2000) Effect of Prey Size on Vulnerability of Copepods to Predation by the Scyphomedusae Aurelia aurita and Cyanea sp. Journal of Plankton Research, 22, 2289-2306.
http://dx.doi.org/10.1093/plankt/22.12.2289 - 3. Vinogradov, F.E. (1968) Vertical Distribution of Oceanic Zooplankton. Academy of Science of the U.S.S.R. Institute of Oceanography. (In Russian, Translated by the Israel Program for Scientific Translation Ltd., Keter Press, Jerusalem, 1970).
- 4. Mills, C.E. (1995) Medusae, Siphonophores, and Ctenophores as Planktivorous Predators in Changing Global Ecosystems. ICES Journal of Marine Science, 52,575-581.
http://dx.doi.org/10.1016/1054-3139(95)80072-7 - 5. Fancett, M.S. and Jenkins, G.P. (1988) Predatory Impact of Scyphomedusae on Icthyoplankton and Other Zooplankton in Port Phillip Bay. Journal of Experimental Marine Biology and Ecology, 116, 63-77.
http://dx.doi.org/10.1016/0022-0981(88)90246-8 - 6. Gómez-Aguirre, S. (1991) Larva éfira y diferenciación de Stomolophus meleagris (Scyphozoa: Rhizostomeae) en plancton de las lagunas costeras de Tabasco, México. Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología, Vol. 62, 383-389.
- 7. Purcell, J.E., Nemazie, D.A., Dorsey, S.E., Houde, E.D. and Gamble, J.C. (1984) Predation Mortality of Bay Anchovy Anchoa mitchilli Eggs and Larvae Due to Scyphomedusae and Ctenophores in Chesapeake Bay. Marine Ecology Progress Series, 114, 47-58.
http://dx.doi.org/10.3354/meps114047 - 8. Purcell, J.E. and Cowan, J.H. (1995) Predation by the Scyphomedusan Chrysaora quinquecirrha on Mnemiopsis leidyi Ctenophores. Marine Ecology Progress Series, 129, 63-70.
http://dx.doi.org/10.3354/meps129063 - 9. Purcell, J.E. and Arai, M.N. (2001) Interactions of Pelagic Cnidarians and Ctenophores with Fish: A Review. Hydrobiologia, 451, 27-44.
http://dx.doi.org/10.1023/A:1011883905394 - 10. Ramírez, E.C. and Zamponi, M.O. (1981) Hydromedusae. In: Boltovskoy, D., Ed. Atlas del Zooplancton del Atlántico Sudoccidental y métodos de trabajo con el zooplancton marino, Publicación especial del Instituto Nacional de Investigación y Pesquero, Mar de Plata, 443-469.
- 11. Zamponi, M.O., Suárez, E. and Gasca, R. (1990) Hidromedusas (Coelenterata: Hydrozoa) y escifomedusas (Coelenterata: Scyphozoa) de la Bahía de la Ascención, Reserva de la Biosfera de Sian Ka′an. In: Navarro, D. and Robinson, J., Eds., Diversidad Biológica de la Reserva de la Biosfera de Sian Kaán, Quintana Roo, México, Centro de Investigaciones de Quintana Roo/Program of Studies in Tropical Conservation, University of Florida, Gainesville, 99-107.
- 12. Pagès, F., White, M.G. and Rodhouse, P.G. (1996) Abundance of Gelatinous Carnivores in the Nekton Community of the Antarctic Polar Frontal Zone in Summer 1994. Marine Ecology Progress Series, 41,139-147.
http://dx.doi.org/10.3354/meps141139 - 13. Ashjian, C.J., Davis, C.S., Gallager, S.M. and Alatalo, P. (2001) Distribution of Plankton, Particles, and Hydrographic Features across Georges Bank Described Using the Video Plankton Recorder. Deep Sea Research, 48, 245-282.
http://dx.doi.org/10.1016/S0967-0645(00)00121-1 - 14. Graham, W.M., Pages, F. and Hamner, W.H. (2001) A Physical Context for Gelatinous Zooplankton Aggregations: A Review. Hydrobiologia, 451,199-212.
http://dx.doi.org/10.1023/A:1011876004427 - 15. Raskoff, K.A., Purcell, J.E. and Hopcroft, R.R. (2005) Gelatinous Zooplankton of the Arctic Ocean: In Situ Observations under the Ice. Polar Biology, 28,207-217.
http://dx.doi.org/10.1007/s00300-004-0677-2 - 16. López-Martínez, J. and álvarez-Tello, J. (2013) The Jellyfish Fishery in Mexico. Agricultural Sciences, 4, 57-61.
http://dx.doi.org/10.4236/as.2013.46A009 - 17. Björnberg, T.K. and Forneris, L. (1956) On the Uneven Distribution of the Copelata of the Fernando Noronha Area. Boletin Instituto Oceanografico São Paulo, 7, 105-111.
http://dx.doi.org/10.1590/S0373-55241956000100006 - 18. Björnberg, T.K. and Forneris, L. (1956) On the Uneven Distribution of the Copelata of the Alcatrazes Area. Boletin Instituto Oceanografico São Paulo, 7, 113-118.
http://dx.doi.org/10.1590/S0373-55241956000100007 - 19. Fenaux, R. (1963) Ecologie et Biologie des Appendiculaires Mediterraneens (Villefranche-sur-mer) Vie et Milieu, 16, 1-142.
- 20. Alldredge, A.L. (1982) Aggregation of Spawning Appendicularians in Surface Windows. Bulletin of Marine Science, 32, 250-254.
- 21. Uye, S. and Ichino, S. (1995) Seasonal Variations in Abundance, Size Composition, Biomass and Production Rate of Oikopleura dioica (Fol) (Tunicata: Appendicularia) in a Temperate Eutrophic Inlet. Journal of Experimental Marine Biology and Ecology, 189, 1-11.
http://dx.doi.org/10.1016/0022-0981(95)00004-B - 22. Flores-Coto, C., Sanvicente-Añorve, L., Vázquez-Gutiérrez, F. and Sánchez-Ramírez, M. (2010) Mesoscale Distribution of Oikopleura and Fritillaria (Appendicularia) in the Southern Gulf of Mexico: Spatial Segregation. Revista de Biología Marina y Oceanografía, 45, 379-388.
http://dx.doi.org/10.4067/S0718-19572010000300002 - 23. Alvariño, A. (1972) Zooplancton del Caribe, Golfo de México y regiones adyacentes del Pacífico. Proceedings of the 4th Congreso Nacional de Oceanografía. México, 17-19 November 1972, 233-247.
- 24. Segura-Puertas, L. (1992) Medusae (Cnidaria) from the Yucatan Shelf and Mexican Caribbean. Bulletin of Marine Science, 51, 353-359.
- 25. Valencia-Correia, K. (1992) Medusas del Golfo de México y litoral norte/noreste de Brasil (ocurrencia y distribución). PhD Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, México.
- 26. Segura-Puertas, L. and Ordóñez-López, U. (1994) Analisis de la comunidad de medusas (Cnidaria) de la región oriental del Banco de Campeche y el Caribe Mexicano. Caribbean Journal of Science, 30, 104-115.
- 27. Correia-Valencia, K. and Segura-Puertas, L. (1995) Nuevos registros de dos especies de Anthomedusae (Pandeidae y Niobiidae) en el Golfo de México. Anales del Instituto de Ciencias del Mar y Limnología, 22, 125-127.
- 28. Suárez-Morales, E., Gasca, R., Segura-Puertas, L. and Biggs, D.C. (2002) Planktonic Cnidarians in a Cold-Core Ring in the Gulf of Mexico. Anales del Instituto de Biología, 73, 19-36.
- 29. Loman-Ramos, L., Ordóñez-López, U. and Segura-Puertas, L. (2007) Variación espacial de la comunidad de medusas del sur del Golfo de México. Hidrobiologica, 17, 203-212.
http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972007000300003&lng=es&nrm=iso - 30. Mendoza-Becerril, M.A., Ocaña-Luna, A., Sánchez-Ramírez, M. and Segura-Puertas, L. (2009) Primer registro de Phialella quadrata y ampliación del límite de distribución de ocho especies de hidromedusas (Hydrozoa) en el Océano Atlántico Occidental. Hidrobiológica, 19, 257-267.
http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972009000300009&lng=es&nrm=is
- 31. Martell-Hernández, L.F. (2010) Variación espacial de la comunidad de cnidarios planctónicos (Medusozoa) en el sureste del Golfo de México (otoño 1998). Master Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico.
- 32. Puente-Tapia, A. (2012) Comunidad de medusas (Cnidaria: Hydrozoa y Scyphozoa) en el sur del Golfo de México (época de secas, 2006). Master Thesis, Posgrado en Ciencias del Mar y Limnología (Biología Marina), Universidad Nacional Autónoma de México, México.
- 33. Bessonov, N., González, O. and Elizarov, A. (1971) Resultados de las investigaciones cubano-soviéticas en el Banco de Campeche. Coloquio sobre Investigaciones y Recursos del Mar Caribe y Regiones Adyacentes, UNESCO, Paris, 318-323.
- 34. Zavala-Hidalgo, J., Morey, S.L. and O′Brien, J. (2003) Seasonal Circulation on the Western Shelf of the Gulf of Mexico Using a High-Resolution Numerical Model. Journal of Geophysical Reearch, 108, 19-36.
http://dx.doi.org/10.1029/2003jc001879 - 35. Salas-de-León, D., Monreal-Gómez, M.A. and Colunga-Enriquez, G. (1992) Hidrografía y circulación geostrófica en el sur de la Bahía de Campeche. Geofísica Internacional, 31, 315-323.
- 36. Signoret, M., Monreal-Gómez, M.A., Aldeco, J. and Salas de León, D. (2006) Hydrography, Oxygen Saturation, Suspended Particulate Matter, and Chlorophyll—A Fluorescence in an Oceanic Region under Freshwater Influence. Estuarine, Coastal and Shelf Science, 69, 153-164.
http://dx.doi.org/10.1016/j.ecss.2006.04.011 - 37. Zavala-García, F., Flores-Coto, C. and Espinosa-Fuentes, M.L. (2016) Relación entre la biomasa zooplanctónica y las descargas de aguas continentales en el sur del Golfo de México (1984-2001). Revista de Biología Marina y Oceanografía, 51, 21-31.
http://dx.doi.org/10.4067/S0718-19572016000100003 - 38. Zavala-Hidalgo, J. and Fernández-Eguiarte, A. (2006) Propuesta para la regionalización de los mares mexicanos desde el punto de vista de los procesos físicos: el Golfo de México. In: Cordova, Y., Vázquez, A., Rosete, F., Enriquez G. and Fernández, B. Eds., Ordenamiento ecológico marino, Instituto Nacional de Ecología-SEMARNAT, México, 21-31.
- 39. White, M.J. (1983) The Measurement of Spatial Segregation. The American Journal of Sociology, 88, 1008-1018.
http://www.jstor.org/stable/2779449 http://dx.doi.org/10.1086/227768 - 40. Buecher, E., Goy, J., Planque, B., Etienne, M. and Dallot, S. (1996) Long Term Fluctuations of Liriope tetraphylla in Villefranche Bay between 1966 and 1993 Compared to Pelagia noctiluca Population. Oceanologica Acta, 20, 143-157.
- 41. Merino-Ibarra, M. (1992) Afloramientos en la plataforma de Yucatán: estructura y fertilización. PhD Thesis, Colegio de Ciencias y Humanidades, Unidad Académica de los Ciclos Profesional y de Posgrado, Universidad Nacional Autónoma de México, México.
- 42. Merino-Ibarra, M. (1997) Upwelling on the Yucatan Shelf: Hydrographic Evidence. Journal of Marine Systems, 13, 101-121.
http://dx.doi.org/10.1016/S0924-7963(96)00123-6 - 43. Colin, S.P., Cotello, J.H., Graham, W.M. and Higgins, J. (2005) Omnivory by the Small Cosmopolitan Hydromedusa Aglaura hemistoma. Limnology and Oceanography, 50, 1264-1268.
http://dx.doi.org/10.4319/lo.2005.50.4.1264 - 44. Ueno, S. and Mitsutani, A. (1994) Small-Scale Swarm of a Hydrozoans Medusa Liripe Tetraphyla in Hiroshima Bay, the Inlad Sea of Japan. Bulletin of Plankton Society of Japan, 41, 165-166.
- 45. Kramp, P.L. (1961) Synopsis of the Medusae of the World. Journal of the Marine Biological Association of the United Kingdom, 40, 7-382.
http://dx.doi.org/10.1017/S0025315400007347 - 46. Arai, M.N. (1992) Active and Passive Factors Affecting Aggregations of Hydromedusae: A Review. Scientia Marina, 56, 99-108.
NOTES
*Corresponding author.






