Open Journal of Internal Medicine
Vol.04 No.03(2014), Article ID:49818,7 pages
10.4236/ojim.2014.43014
Rectus Sheath Hematoma—A Rare and Dangerous Complication of Anticoagulation Therapy
—A Case Report and Review of Current Literature
Tobias Hoefflinghaus1, Lea Landolt2, Esther B. Bachli3*
1Medical Department, City Hospital Waid, Zurich, Switzerland
2Clinic and Policlinic of Internal Medicine, University Hospital Zurich, Zurich, Switzerland
3Department of Internal Medicine, Uster Hospital, Uster, Switzerland
Email: *esther.baechli@spitaluster.ch
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 14 July 2014; revised 13 August 2014; accepted 7 September 2014

ABSTRACT
Rectus sheath hematoma (RSH) is a rare bleeding site which can be life-threatening. Our aim was to analyze clinical features, diagnosis, treatment and outcome of these cases in a general hospital. Results: During a period of 24 months, 8 cases of RSH were diagnosed (0.1% of all inpatients). Mean age was 79.4 years (y, SD +/− 14.1). 75% were female. 7 patients (pts) were on therapy with oral anticoagulants (OAC), 2 had a history of additional therapy with aspirin (ASA), 4 had low molecular weight heparin (LMWH) for bridging of OAC therapy. 4 pts had INR values between 3.1 - 4.4. One pt with liver cirrhosis (Child A), mild thrombocytopenia had ASA and LMWH prophylactically. Five pts (63%) were coughing. Main symptom was localized pain in 7 pts. One pt was incidentally diagnosed on a CT scan performed for different reason. 7 pts had a palpable mass of the abdominal wall. 5 RSH were diagnosed with CT, 2 with ultrasound, 1 clinically. All pts were treated conservatively. In 7 pts vitamine K and Beriplex® was substituted to reverse OAC. 4 pts received blood transfusions (1 - 4 units). The mean change of hemoglobin (Hb) was 37 g/l (SD +/− 22 g/l). The renal function was impaired in 7 pts (mean creatinine clearance 51 ml/min +/− 12.4) and declined further. 3 out of 8 pts died (38%, age 90 - 92 y, all female). In these patients, the observed change in Hb was 42 - 58 g/l. 2 pts had combination of VKA and LMWH. 1 pt had VKA only. Conclusion: Acute abdominal pain in pts with any form of OAC or therapy to inhibit platelet function should always raise suspicion about RSH. Extensive blood loss, shock and aggravation of renal failure can be fatal. In majority of cases, the hematoma is palpable and should prompt further examination of the abdominal wall. It is crucial to normalize coagulation parameters and to correct intravascular volume depletion to avoid shock and further decline of renal failure.
Keywords:
Rectus Sheath Hematoma, Anticoagulation, Complication

1. Introduction
In 2012, more than 6 million patients in the USA received an antithrombotic therapy with oral anticoagulants [1] (OAC). Most physicians care for patients who are treated with oral anticoagulants. Despite the marked benefits of this therapy in the prevention of thromboembolic events, a bleeding risk remains. Risk models have been developed to detect patients at increased bleeding risk for certain clinical conditions, such as the HAS-BLED score [2] for patients with atrial fibrillation of the European Society of Cardiology. One of the severe and probably rare bleeding complications is the rectus sheath hematoma (RSH). This complication of anticoagulant therapy increases the morbidity, even mortality and the length of stay in hospitalized patients [3] . The precise prevalence is unknown and there are only case reports or small case series reported [3] - [6] . We report the prevalence of this complication and potential risk factors in our hospital in a period of 24 months.
2. Methods
Over a 24 month period, we analyzed retrospectively charts of medical inpatients with rectus sheath hematoma in our department. Clinical characteristics, co-medication, laboratory values, applied therapy and in-hospital outcome were analyzed.
Due to the small sample size, we abstained from performing a statistical analysis.
The CT imaging of rectus sheath hematoma was classified according to Berna et al. [7] . Grade I is defined as mild RSH (intramuscular, unilateral, no dissection along fascia), grade II is moderate RSH (intramuscular, dissection along fascia, possible bilateral involvement but no extension into prevesical space) and grade III is severe RSH (dissection along fascia, extension into peritoneum and prevesical space).
3. Results
Over a period of 24 months, 8 cases of RSH were observed. This accounts for 0.1% (8 of 7450) of patients hospitalized on the medical ward in this time period. The patients with RSH had a mean age of 79.4 years (+/−14.1 years) and 75% were female. The mean age of the patients treated in this period was 64 years and 55% were female.
3.1. Reason for Hospitalization in Patients with RSH
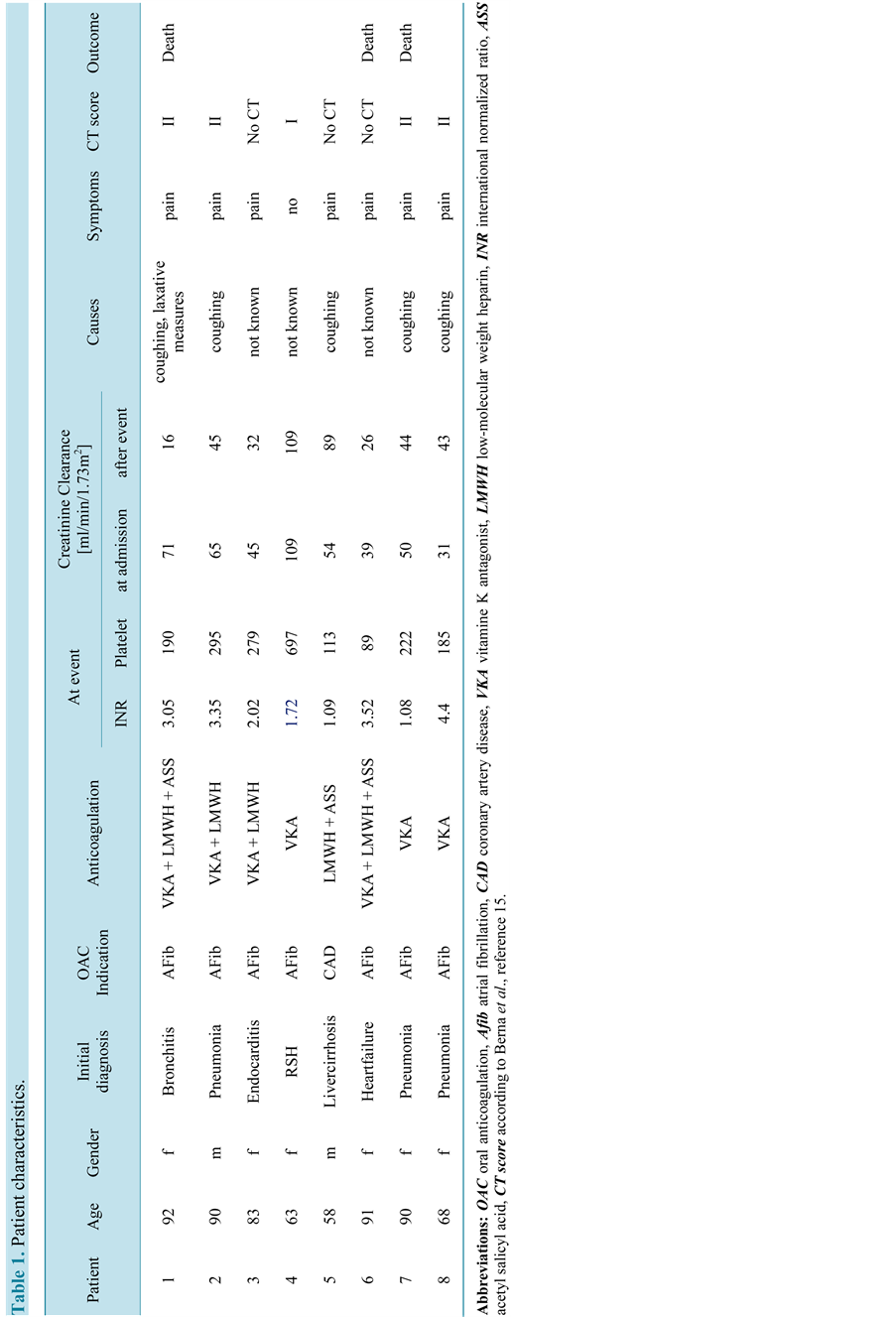
4 out of 8 patients were hospitalized for respiratory infections (pneumonia in 3 cases and bronchitis in one; see Table 1). One patient had endocarditis, one symptomatic heart failure and one had liver cirrhosis with anemia. In the remaining patient, RSH was diagnosed incidentally in a CT scan. This was the only patient hospitalized for primary diagnosis of RSH. The patient had a history of pyelonephritis and sudden backpain radiating to the groin, which was the indication for the CT scan. The patient was on OAC therapy for atrial fibrillation and the INR value was 1.72 at the time of the diagnosis of RSH. The clinical examination of the abdominal wall was unremarkable and the patient denied pain in the region of RSH.
3.2. Indication for OAC Therapy
7 patients were on OAC therapy because of atrial fibrillation and a CHA2DS2VASc score above one. 4 of the 7 patients were on therapeutic dose of low molecular weight heparin (LMWH) to bridge OAC therapy while not in the therapeutic range. Of those 4 patients, two patients had additionally ASA therapy (100 mg daily). One patient received ASA because of atrial fibrillation. In this patient, ASA therapy was stopped and LMWH and OAC according to the ESC guidelines were started. The RSH was diagnosed 4 days after the ASA therapy was stopped. The second patient was on ASA therapy as an outpatient. Initially, it was prescribed as a primary prophylactic therapy in heart failure. After diagnosis of atrial fibrillation ASA therapy was stopped, LMWH and
Table 1.Patient characteristics.
OAC was prescribed, the RSH occurred 7 days after the ASA therapy was stopped. No other therapy which could interfere with platelet function was given in any of the patients. The remaining 3 patients were on OAC monotherapy. Only one patient developed RSH without OAC therapy. He had liver cirrhosis (Child-Pugh score A) and was on ASA therapy because of stable coronary artery disease without any stents or intervention in the 12 months before the current hospitalization. He received LMWH in a prophylactic dose (5000 U Dalteparins.c. once daily, application into the leg) and ASA therapy in a dose of 100 mg daily. He was hospitalized because of coughing and nose bleeding before the hospitalization and to assess a severe anemia of 80 g/L. During the hospitalization he had no bleeding tendency, his platelet count was 113.000/ml and his INR was 1.09. His anemia was due to iron deficiency without any signs of acute bleeding.
3.3. Coagulation Studies or INR Levels
The patient with liver cirrhosis had a normal INR, mild thrombocytopenia and ASA therapy. In 2 of the 7 patients with OAC therapy, the INR was below 2 (1.0 and 1.72, resp.), none of these patients had bridging therapy with LMWH s.c. at the time of diagnosis of the RSH. In one of the 7 patients on OAC the INR was in the therapeutic range for the first time (INR 2.0) and LMWH was continued for another 24 hours. 4 of the 7 patients on OAC had an INR value between 3.0 - 4.4. 3 of them were still on bridging therapy with LMWH and the patient with an INR of 4.4 had no bridging therapy with LMWH.
3.4. Renal Function and LMWH
All but one patient had an impaired renal function according to creatinine clearance estimated with the Cockroft- Gault-formula (mean GFR 58 ml/min/1.73m2, range 31 - 109 ml/min/1.73m2; see Table 1). The 3 patients with fatal outcome had a further decrease of the renal function due to prerenal impairment due to the RSH. The dose of the LMWH had not to be adjusted to the impaired renal function and none of the patient had LMWH therapy for more than 5 days.
3.5. Possible Causes and Symptoms of RSH
In 5 of the 8 patients (63%) coughing was observed or reported by the patient or nurses. In three patients none of the in the literature proposed risk factors for RSH could be found. In these 3 patients no previous traumatic event such as a fall or accident, recent labor, femoral artery puncture or abdominal surgery could be established. None of these 3 patients had subcutaneous injections into the abdominal wall for instance for insulin applications or for LMWH. None of the 8 patients had marks of accidental subcutaneous injections in the abdominal wall on close inspection. One of the 5 patients with cough had additionally severe constipation with laxative measures, which might have added to the RSH.
All patients but one with the incidental finding described localized severe abdominal pain. Seven patients had a palpable mass of the abdominal wall. Five patients had developed RSH in lower abdomen, 2 in the hemi abdomen and only one patient had RSH in the upper abdomen. In one patient the precise localisation of the hematoma could not be clinically assessed because of severe obesity (BMI 36.2 kg/m2). Five RSH were diagnosed with computed tomography, 2 with abdominal ultrasound, one clinically by ecchymosis of the skin and a palpable mass (see Figure 1).
3.6. Treatment of RSH and Clinical Outcome
All patients were treated conservatively. In 7 patients, vitamin K and a 4-factor concentrate (Beriplex®) were given to normalize the INR. In the asymptomatic patient only vitamin K was substituted because there was no clinically or radiological sign of active bleeding and small extent of RSH.
In 5 patients, a hemorrhagic shock was diagnosed. The mean drop of hemoglobin was 37 g/L (+/−22 g/L) in all patients in 24 h, despite treatment with vitamin K and 4-factor concentrates. The change of hemoglobin was higher in the patients who died (42 - 58 g/L). 4 patients, who had a hemoglobin concentration below 80 g/L, received packed red blood cells (1 - 4 units). No platelet transfusions were given, possibly due the long interval between stopping ASA therapy and development of the RSH. The renal function was chronically impaired in 7 patients before the event (estimated glomerular filtration rate 51 ml/min +/− 12.4 ml/min) and declined further in these patients due to the hemorrhagic shock. No renal replacement therapy was administered. Three out of 8
Figure 1. Shows the computed tomography of a 90-year-old male patient who was hospitalized for pneumonia (patient 2 in Table 1). Note the large RSH in the musculus rectus abdominis on the left (asterisk) and the thin muscle layer on the right (arrow). The patient received LMWH overlapping to OAC due to subtherapeutic INR levels. The INR at event was 2.0, thrombocyte count was normal. He received despite Vitamine K a 4-factor concentrate to optimize coagulation patterns. The baseline kidney function was mildly reduced and dec- lined to stage III renal failure. After complete recovery with con- servative therapy he could be discharged at home.
patients died (38%). These patients were 90 - 92 years old, female and had chronic renal failure with a mean GFR of 53 ml/min/1.73 m2 (range 39 - 71 ml/min/1.73 m2). The observed change in hemoglobin was 42 - 58 g/L. Two patients were on OAC and LMWH bridging therapy (INR 3.1 and 3.5, resp.). One patient was on OAC with an INR of 1.1. An autopsy was not performed. The cause of death was progressive renal failure with metabolic acidosis in two patients and heart and renal failure in one patient. None of these patients received intensive care with renal replacement therapy according to the wishes of these elderly patients.
4. Discussion
Our case series shows a substantial mortality (38%; 3 out of 8 patients) and morbidity of RSH in an unselected group of medical inpatients in a general hospital. Mortality rates vary in different reports between 0% [8] and 20% [5] . To date the largest series was reported by Cherry et al. in 2006 [3] . They found a mortality rate of 1.6% (2/126 patients). Their patients were younger than in our report (mean age 67.9 vs. 79.4 years). One patient had a coagulation disorder (hemophilia A) and the other had a sustained bleeding despite optimal hemostatic treatment with fatal outcome. The high mortality in our report is—to our opinion—caused by the small sample size, the higher age of our patients with RSH, relevant comorbidities like chronic renal failure, use of bridging therapy and patients who did not wish to receive renal replacement therapy or treatment on an ICU. In our series, ASA therapy was stopped at least one week before the bleeding event in 2 of the 3 patients. One patient had ASA, a mild thrombocytopenia and liver cirrhosis stage Child A with a preserved coagulation and no OAC. In one study, the combination of ASA and LMWH was considered as problematic due to the prolonged pharmacological action leading in this study to more interventional treatment [9] . One could postulate that the use of the combination of OAC and inhibition of platelet function might be associated with an increase of RSH. We could not find any reports to support this postulate.
The elderly patients might be more vulnerable to develop RSH. This may be caused by abdominal tissue atrophy and sarcopenia, vascular fragility and less external compression of the surrounding tissue during bleeding [10] . In elderly patients renal function often is reduced, which might lead to accumulation of LMWH. In our patients, the renal function was impaired, but not as severe to affect the elimination of the LMWH.
In Switzerland, several observations initiated by a group of medical clinics lead to the observation that injections of prophylactic doses of LMWH in the abdominal wall might be associated with the occurrence of RSH [11] . These observations, which are confidentially published as a quality measure by the board of internists in Switzerland, changed the application of LMWH in Switzerland. In none of the patients reported in this observational study the LMWH was injected into the abdominal wall.
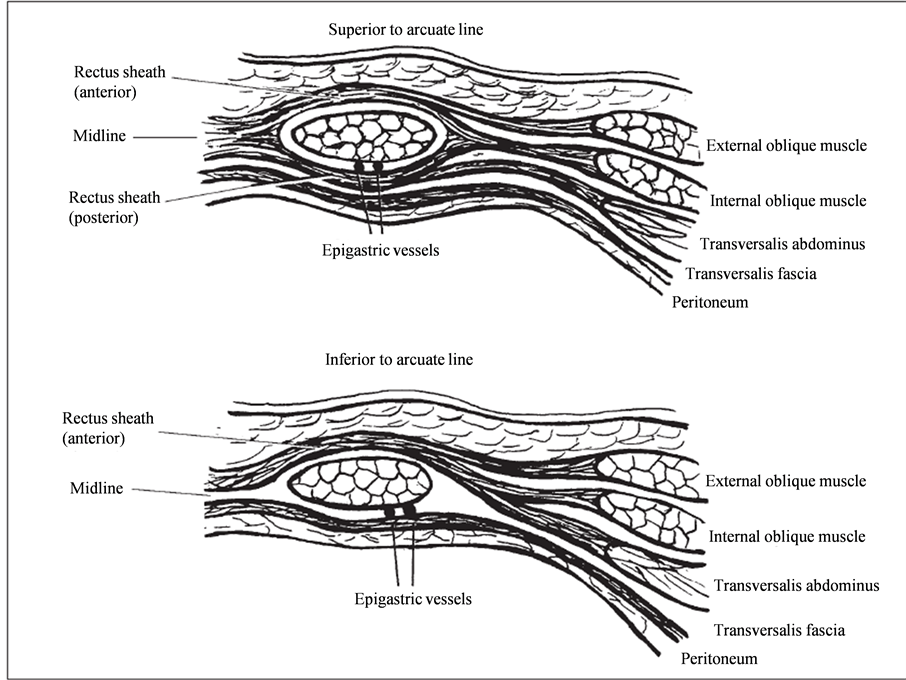
Despite the fact that anticoagulation therapy is a systemic therapy, we only found isolated rectus sheath hematomas without any other signs of different bleeding sites (e.g. mucosal, cutaneous or gastrointestinal bleeding). The site of bleeding is an important issue because of the anatomy of the rectus sheath. The posterior wall of the musculus rectus is protected against sheer or other injuries by the aponeurosis of muscles and the fascia transversalis. Therefore, epigastric vessels are enclosed and protected above the linea arcuata (Figure 2). Below this line, there is no such barrier and the vessels might be vulnerable to injuries during strenuous exercise of the abdominal muscles. This is the case during coughing, defecation, abdominal trauma, surgery and labor. Due to the lack of protection of the posterior aponeurosis, hemorrhage can extend into the pelvis where surrounding tissue might not be able to compress the bleeding site [3] [10] [12] [13] . This is the possible explanation why anticoagulants as a systemic therapy increase the risk of RSH under special circumstances described above.
Coughing was present in the majority of our patients (63%, 6 out of 8 patients). This was observed in other reports [3] [10] .
Women seem to be more vulnerable to develop RSH. This might be due to the lower muscle mass of rectus abdominis muscle in females [3] [4] [9] [14] and former pregnancies and labor [15] .
Figure 2. From Edlow JA et al. [13] with kind permission of Elsevier Limited provided by Copyright Clearance Center. Upper image shows the rectus sheath anatomy above the arcuate line with posterior protection of the epigastric vessels which is formed by the aponeurosis of muscle fibers of the internal oblique and the transversalis abdominus muscle and the transversal fascia. In the lower image, the posterior rectus sheath is missing, leaving the epigastric vessels unprotected. A bleeding at this site can extend to the pelvis and can cause severe loss of blood.
As in many other reports, the typical clinical features in our study were localized abdominal pain and a palpable abdominal mass. The Fothergill’s sign consists of a tender abdominal mass that is restricted to one side and does not disappear during contraction of the rectus muscle. The Carnett’s sign describes tenderness within the abdominal wall at rest and during head rise [15] . The diagnosis can be made clinically or with imaging modalities like sonography or computed tomography [7] where the extent of hemorrhage can be better visualized and a grading system can be applied [7] . CT imaging offers the advantage of defining the extent of bleeding and might localize a culprit vessel which could be embolized. Ultrasonography offers good resolution but application might be limited in patients with massive abdominal pain or abdominal obesity. To our knowledge, there is no clinical scoring system incorporating imaging findings and related outcome. Berna et al. found in a small series that all grade III hematomas required blood transfusions [16] . All hematomas (grade I-III) could be treated conservatively.
Rarely RSH is found incidentally, as in one patient in our series. Lohle et al. [17] described 3 patients who were examined for suspected appendicitis. They found small RSH which were confirmed by CT. Only one patient was on OAC therapy. Therefore, in patients with abdominal pain the abdominal wall should be examined carefully and the rare but potentially fatal diagnosis of RSH must be considered.
In our series, bleeding was controlled in 5 of 8 with medical interventions only. We performed no surgery or vessel embolization in our patients. This was due to the patient comorbidities and their will not to undergo any invasive treatment. Unfortunately, specific guidelines or randomized trials or registries addressing the management of rectus sheath hematoma are lacking. Therefore, the patient management is based on anecdotal reports, small case series and personal experience. According to the severity of bleeding and its associated complications we recommend complete reversal of the anticoagulant or antithrombotic therapy under careful consideration of the primary indication of these therapies. In 2012 two cases were described [18] in which RSH lead to an abdominal compartment syndrome with renal failure and complete recovery after surgical decompression. After optimization of coagulation and surgical decompression with drainage of the hematoma these patients recovered. Other reports showed that a conservative approach is possible in the majority of the patients [3] [4] [6] . A percutaneous approach of vessel embolization is possible and should be evaluated in patients with hemodynamic compromise or unstoppable bleeding despite adequate measures to control the bleeding by fluid resuscitation and reversal of anticoagulation [19] . Surgical therapy or embolisation of the bleeding vessel might be no option in elderly frail or multimorbid patients.
If the selective Factor Xa or Factor II inhibitors, the “novel oral anticoagulants (NOAC)”, will have any advantage in this setting is unknown. In the published trials RSH was not specifically reported in the patients on vitamin k antagonists or patients on any of the NOAC [20] . Major bleeding was reported in all groups, but RSH was not specifically mentioned. To date there are no specific antidotes to reverse the action of NOACs. This might be of concern because these new OAC are to great extent excreted renally and it is known that renal impairment prolongs their half life considerably.
5. Conclusion
Acute abdominal pain in patients under anticoagulant and/or antiaggregation therapy combined with or without renal impairment should always raise suspicion about rectus sheath hematoma. Extensive blood loss, shock and aggravation of renal failure can be fatal. In the majority of cases, the hematoma is palpable and should prompt a CT scan of the abdomen. It is crucial to normalize coagulation parameters, to correct intravascular volume depletion to avoid shock and further decline of renal failure. In cases of inappropriate response to therapy, surgery or vessel embolization should be considered.
References
- Roger, V.L., Go, A.S., Lloyd-Jones, D.M., Benjamin, E.J., Berry, J.D., Borden, W.B., et al. (2012) Heart Disease and Stroke Statistics—2012 Update: A Report from the American Heart Association. Circulation, 125, e2-e220. http://dx.doi.org/10.1161/CIR.0b013e31823ac046
- Task Force European Society of Cardiology (2010) Guidelines for the Management of Atrial Fibrillation. European Heart Journal, 31, 2369-2429. http://dx.doi.org/10.1093/eurheartj/ehq278
- Cherry, W.B. and Mueller, P.S. (2006) Rectus Sheath Hematoma: Review of 126 Cases at a Single Institution. Medi- cine, 85, 105-110. http://dx.doi.org/10.1097/01.md.0000216818.13067.5a
- Ahmet, D., Özcan, T., Türkmenoglu, Ö., Colak, T., Karaca, K., Canbaz, H., et al. (2011)Spontaneous Rectus Sheath Hematoma in Patients on Anticoagulation Therapy. Turkish Association of Trauma and Emergency Surgery, 17, 210- 214.
- Carkman, S., Özben, V., Zengin, K., Somuncu, E. and Karatas, A. (2010) Spontaneous Rectus Sheath Hematoma: An Analysis of 15 Cases. Turkish Association of Trauma and Emergency Surgery, 16, 532-536.
- De Martino, C., Martino, A., Giamattei, R.M., Viola, G., Pisapia, A. and Fatigati, G. (2011) Spontaneous Rectus Sheath Hematoma: A Rare Condition with Uneasy Diagnosis and Multidisciplinary Treatment: Report of 5 Cases and Review of Literature. Annali Italiani di Chirurgia, 82, 399-404.
- Berna, J.D., Garcia-Medina, V., Guirao, J. and Garcia-Medina, J. (1996) Rectus Sheath Hematoma: Diagnostic Classification by CT. Abdom Imaging, 21, 62-64. http://dx.doi.org/10.1007/s002619900011
- Buffone, A., Basile, G., Costanzo, M., Veroux, M., Terranova, L., Basile, A., et al. (2013) Management of Patients with Rectus Sheath Hematoma: Personal Experience. Journal of the Formosan Medical Association. http://dx.doi.org/10.1016/j.jfma.2013.04.016
- Smithson, A., Ruiz, J., Perello, R., Valverde, M., Ramos, J. and Garzo, L. (2013) Diagnostic and Management of Spontaneous Rectus Sheath Hematoma. European Journal of Internal Medicine, 24, 579-582. http://dx.doi.org/10.1016/j.ejim.2013.02.016
- Macias-Robles, M.D., Peliz, M.G. and Gonzalez-Ordonez, A.J. (2005) Prophylaxis with Enoxaparin Can Produce a Giant Abdominal Wall Haematoma When Associated with Low Doses of Aspirin among Elderly Patients Suffering Cough Attacks. Blood Coagulation Fibrinolysis, 16, 217-219. http://dx.doi.org/10.1097/01.mbc.0000164433.43911.4f
- www.komplikationsliste.ch
- Titone, C., Lipsius, M. and Krakauer, J.S. (1972) Spontaneous Hematoma of the Rectus Abdominis Muscle: Critical Review of 50 Cases with Emphasis on Early Diagnosis and Treatment. Surgery, 72, 568-572.
- Edlow, J.A., Juang, P., Margulies, S. and Burstein, J. (1999) Rectus Sheath Hematoma. Annals of Emergency Medicine, 34, 671-675. http://dx.doi.org/10.1016/S0196-0644(99)70172-1
- Teske, J.M. (1946) Hematoma of the Rectus Abdominis Muscle: Report of a Case and Analysis of 100 Cases from the Literature. The American Journal of Surgery, 71, 689-695. http://dx.doi.org/10.1016/0002-9610(46)90453-9
- Osinbowale, O. and Bartholomew, J.R. (2008) Rectus Sheath Hematoma. Vascular Medicine, 13, 275-279. http://dx.doi.org/10.1177/1358863X08094767
- Berna, J.D., Zuazu, I., Madrigal, M., Garcia-Medina, V., Fernandez, C. and Guirado, F. (2000) Conservative Treatment of Large Rectus Sheath Hematoma in Patients Undergoing Anticoagulant Therapy. Abdom Imaging, 25, 230-234. http://dx.doi.org/10.1007/s002610000007
- Lohle, P.N., Puylaert, J.B., Coerkamp, E.G. and Hermans, E.T. (1995) Nonpalpable Rectus Sheath Hematoma Clinically Mascerading as Appendicitis: US and CT Diagnosis. Abdom Imaging, 20, 152-154. http://dx.doi.org/10.1007/BF00201526
- McBeth, P.B., Dunham, M., Ball, C.G. and Kirkpatrick, A.W. (2012) Correct the Coagulopathy and Scoop It out: Complete Reversal of Anuric Renal Failure through the Operative Decompression of Extraperitoneal Hematoma-Induced Abdominal Compartment Syndrome. Case Reports in Medicine, 2012, Article ID: 946103. http://dx.doi.org/10.1155/2012/946103
- Rimola, J., Perendreu, J., Falco, J., Fortuno, J.R., Massuet, A. and Branera, J. (2007) Percutaneous Arterial Embolization in the Management of Rectus Sheath Hematoma. American Journal of Roentgenology, 188, W497-W502. http://dx.doi.org/10.2214/AJR.06.0861
- Van der Hulle, T., Kooiman, J., Den Exter, P.L., Dekkers, O.M., Klok, F.A. and Huisman, M.V. (2013) Effectiveness and Safety of Novel Oral Anticoagulants as Compared with Vitamine K Antagonist in the Treatment of Acute Symptomatic Venous Thromboembolism: A Systematic Review and Meta-Analysis. Journal of Thrombosis and Hemostasis, 12, 320-328.

*Corresponding author.