Advances in Bioscience and Biotechnology
Vol. 4 No. 3 (2013) , Article ID: 28775 , 8 pages DOI:10.4236/abb.2013.43047
Attenuation of experimentally induced diabetic neuropathy in association with reduced oxidative-nitrosative stress by chronic administration of Momordica charantia
![]()
1Department of Pharmacology, ISF College of Pharmacy, Moga, India
2Department of Pharmaceutical Sciences, Univeristy of Kashmir, Srinagar, India
Email: *zafar18@rediffmail.com, n.tabassum.uk@gmail.com, plsharma.isf@gmail.com
Received 6 January 2013; revised 16 February 2013; accepted 28 February 2013
Keywords: Diabetes; Momordica charantia; Oxidative-Nitrosative Stress; Neuropathy; Pain
ABSTRACT
Momordica Charantia (MC) is one of the most famous traditional plant worldwide, used for the treatment of diabetes and its complications. In the present study possible protective effect of MC in Streptozotocin (STZ) induced diabetic neuropathy in mice was evaluated. STZ induced diabetic mice were orally administered MC at various doses (200 - 800 mg/kg) for six weeks. Diabetes induced neuropathic pain was assessed by hot plate test, formalin test and tail flick test at the beginning and end of the study. Serum TBARS, NO and SOD levels were estimated at the end of the study as the markers of oxidative-nitrosative stress. Rota rod test was employed to assess the effect of treatment on motor coordination. The results showed that STZ induced diabetes significantly decreased the pain threshold as was indicated by increased flinching in formalin test and decreased withdrawal latency in hot plate and tail flick tests. Oxidative-nitrosative stress was significantly increased in diabetic animals. Chronic administration of MC significantly attenuated diabetes induced increase in flinches and decrease in withdrawal latency without impacting sensory and motor functions. MC administration also exhibited dose dependant reduction of hyperglycemia and serum TBARS, NO and SOD levels in diabetic mice. The results suggest that long term use of MC protects against diabetes induced neuropathy in association with attenuation of hyperglycemia and oxidative-nitrosative stress.
1. INTRODUCTION
Diabetic neuropathy remains an important clinical problem affecting more than half of all diabetic patients and is a leading cause of nontraumatic amputations and autonomic failure [1,2]. Diabetes induced neuropathic pain is much more difficult to treat than nociceptive pain [3]. Various classes of drugs such as nonsteroidal anti-inflammatory drugs, antidepressants and anticonvulsants have partial utility because of their limited effectiveness and severe potential toxicity [4]. Opoids are effective antinociceptive drugs; however, their antinonciceptive activity decreases in diabetes associated neuropathic pain [5]. The development of safe and effective drugs for prevention and treatment of diabetes-associated neuropathy is, therefore, highly warranted.
The pathogenesis of diabetic neuropathy still remains unclear although a number of mechanisms have been implicated which include increased aldose reductase (AR) activity, nonenzymatic glycation, activation of protein kinase C, and impaired neurotrophic support. These mechanisms contribute to increased production of reactive oxygen species (ROS) and nitrogen species (RNS), insufficient up-regulation or down-regulation of antioxidative defense system [6-8]. Enhanced oxidative-nitrosative stress and changes in antioxidant capacity, observed in both clinical and experimental diabetes mellitus, are considered to be the etiology of chronic diabetic complications [9]. However, strict glycemic control and use of antioxidants slows or even reverses the progression of diabetic neuropathy [9,10]. We have recently shown that standardized freeze dried powder of Momordica charnatia (MC) having antihyperglycemic activity coupled with strong antioxidant property is responsible for neuroprotective activity in diabetic mice [11]. MC is one of the most popular anti-diabetic plants worldwide [12] and has been referred to as “vegetable insulin” in Asia, Africa and latin America [13]. Apart from diabetes, MC fruit extracts are also clinically used in conditions like dyslipidemia, microbial infections, and potentially as a cytotoxic agent for various types of cancer [14]. Various biologically active chemicals reported from MC include glycosides, saponins, alkaloids, fixed oils, triterpenes, polyphenols, proteins and steroids. The beneficial effects of MC in diabetes are attributed to active constituents like charantin, insulin-like peptide (plant (p)-insulin), cucurbutanoids, momordicin, and oleanolic acids [14,15] while as antioxidant activity is mainly due to its total phenolic and ascorbic acid content [16].
The aforementioned literature survey reveals that MC possesses antioxidant, antihyperglycemic and neuroprotective activity but no detailed work has ever been carried out to evaluate protective effect of MC on diabetes induced neuropathy. Although, an earlier report suggest the ameliorative effect of MC in diabetic neuropathy [17] but the study was done with single dose using one nociception model and the mechanism of action was not investigated. Therefore, the current study aimed to evaluate the effects of MC, as a dietary antioxidant, on diabetes-induced neuropathy in mice using various thermal and chemical models of nociception.
2. MATERIALS AND METHODS
2.1. Plant Material and Preparation of Freeze Dried Powder of MC
Fresh green fruit of MC were purchased from local market and were identified and authenticated by the Department of Botany, Punjab University, Chandigarh. Voucher specimen (PH-2009/0069) was deposited in the herbarium of the institute. After washing thoroughly juice of whole fruit was prepared on a juicer (Philiphs, India), passed through a coarse sieve and completely lyophilized by continuous freeze drying operation for 24 h. The yield was ~6.3 g powder/100g of fresh juice. The powder so obtained was stored in airtight container at low temperature and suspended in 0.5% carboxy methylcellulose (CMC) before oral administration.
2.2. Standardization of Freeze Dried Powder of MC
Phytochemical screening of the MC was carried out employing standard procedures and tests [18]. Further, the antioxidant components of MC were standardized on the basis of total phenolic content and ascorbic acid as previously described [11].
2.3. Drugs and Chemicals
STZ (Sigma, USA) was dissolved in 0.1 N citrate buffer prepared by mixing 0.1 N citric acid solution and 0.1 N trisodium citrate solution. Insulin (Knoll Pharmaceuticals, India) was dissolved in normal saline and Lycopene (Sigma, USA) was first dissolved in Tween 80 (5%) and then in normal saline. All other chemicals employed in the present study were of analar grade.
2.4. Animals
Male Swiss albino mice weighing 22 - 30 g were procured from the animal facility of the Institute and maintained on standard laboratory diet (Kissan Feeds Ltd., Mumbai, India) and tap water ad libitum. The animals were housed in standard polypropylene cages (5 mice/ cage) and maintained under controlled room temperature (25˚C ± 2˚C) with 12:12 h light and dark cycle. The guidelines of committee for the purpose of control and supervision of experiments on animals (CPCSEA), Govt. of India were followed and prior permission was sought from the institutional animal ethics committee for conducting the study.
2.5. Experimental Diabetes in Mice
The mice were rendered diabetic by a single injection of streptozotocin (STZ) (200 mg/kg, i.p.) dissolved in 0.1 N citrate buffer at pH 4.5 [19]. Age matched mice were injected with 0.1 N citrate buffer to serve as control. Blood samples for glucose estimation were obtained from tail vein, 72 hrs after the administration of STZ. Mice with a blood glucose level of more than 250 mg/dl were considered to be diabetic and included in the study. Serum glucose was estimated spectrophotometrically by using commercially available kits (J. Mitra & Co. Ltd. New Delhi, India). Body weight was recorded at the start and end of the treatments.
2.6. Experimental Design and Treatment Schedule
Animals were divided into seven groups (n = 10) and each group received different treatment for 6 weeks. Group one and two were normal and diabetic control, respectively, which received 0.5% CMC orally for six weeks. Third, fourth and fifth groups of diabetic mice, orally received 200, 400 and 800 mg/kg dose of MC daily once for 6 weeks, respectively. Groups sixth and seventh of diabetic mice received insulin (8 IU/Kg of porcine zinc insulin suspension and 3 IU/Kg of porcine regular insulin s.c., b.i.d.) and lycopene (5 mg/kg, p.o., o.d) for 6 weeks, respectively. Each animal was subjected to different behavioral assays at the start (0 week) and at the end of the six weeks one hour after drug/vehicle administration. Serum nitrite/nitrate, TBARS, SOD and glucose were measured at the end of 6th week. The doses of MC or lycopene and Insulin employed in the present study were taken from pilot studies using serum nitrite/nitrate and glucose level, respectively, as the end point.
2.7. Behavioral Assays
2.7.1. Hot Plate Test
In this test animals were placed individually on a hot plate (Eddy’s hot plate) with the temperature adjusted to 55˚C ± 1˚C [20]. The latency to the first sign of paw licking or jump response to avoid the heat was taken as the index of pain threshold; the cut off time was 10 sec in order to avoid the damage to the paw.
2.7.2. Formalin Test
Each animal was administered 0.05 mL of 10% formalin into the dorsal portion of the front paw. Each individual animal was placed into a clear plastic cage for observation. Readings are taken at 30 and 60 min and scored according to a pain scale. Pain responses are indicated by elevation or favoring of the paw or excessive licking and biting of the paw. Analgesic response or protection is indicated if both paws are resting on the floor with no obvious favoring of the injected paw [21].
2.7.3. Tail Flick Test
The nociceptive threshold in mice was determined using withdrawal latency in tail flick test [22] using tail flick analgesiometer (INCO Pvt. Ltd., Ambala, India). The lower 5 cm of tail of each mouse was exposed to radiant heat given by a nichrome wire. The intensity of the radiant heat was adjusted to obtain a basal or pre-treatment latency of 6 - 8 seconds in both diabetic and non-diabetic animals. Tail-Flick latency is time interval taken by mice to flick its tail after exposure to a source of radiant heat. Maximum cut off latency time was fixed at 10 seconds to avoid the damage to the tissue.
2.7.4. Rota-Rod Test
Rota rod was used to evaluate motor coordination by testing the ability of mice to remain on a revolving rod [23]. The difference in the fall off time from the rotating rod between the control and treated animals was taken as an index of motor incoordination. Each mouse was given four or five trials before the actual reading was taken.
2.8. Assessment of Oxidative-Nitrosative Stress
2.8.1. Estimation of Serum Nitrite/Nitrate
To serum sample (100 μl) was added carbonate buffer (pH 9.0, 400 μl) followed by addition of small amount (~0.15 g) of copper-cadmium alloy. The tubes were incubated at room temperature (1 h) to reduce nitrate to nitrite. The reaction was stopped by adding 0.35 M sodium hydroxide (100 μl). Following this, 120 mM of zinc sulfate solution (400 μl) was added to deproteinate the serum samples. The samples were allowed to stand (10 min) and then centrifuged at 4000 g (10 min). To aliquots (500 μl) of clear supernatant was added 500 μl of Greiss reagent (a 1:1 mixture of 1% sulphanilamide in 5% H3PO4 and 1% N-[1-naphtyl]-ethylenediamine) and After 10 minute incubation period serum nitrite/nitrate was measured spectrophotometrically (UV 1700, Shimadzu) at 545 nm [24]. The nitrite/nitrate concentrations in the samples were calculated from a standard curve of sodium nitrite (0.5 - 40 μM).
2.8.2. Estimation of Serum Thiobarbituric Acid Reactive Substances (TBARS)
1mL of trichloroacetic acid (20%) and 1mL of thiobarbituric acid reagent (1%) was added to 100 μL of serum and then mixed and incubated at 100˚C (30 min). After cooling on ice, samples were centrifuged at 1000 g (20 min). Serum concentration of TBARS was measured spectrophotometrically at 532 nm. A standard graph using 1,1,3,3 tetramethoxypropane (1 - 50 μM) was plotted to calculate the concentration of TBARS [25].
2.8.3. Estimation of SOD Activity
SOD activity was assessed in the serum based on the method of inhibiting auto-oxidation of epinephrine [26]. Auto-oxidation of epinephrine was initiated by adding Fenton reagent (200 µL) to a mixture of epinephrine (300 µM), Na2CO3 (1 mM), EDTA (10 mM), and 200 µL of deionized water or serum at a final volume of 1.2 ml. The auto-oxidation was read in a spectrophotometer at 480 nm every 30 sec for 5 min. A graph of absorbance versus time was plotted and the initial rate of auto-oxidation calculated. One unit of SOD activity was defined as the concentration of the enzyme in the plasma that caused 50% reduction in the auto-oxidation of epinephrine [27].
2.9. Statistical Analysis
All values are expressed as mean ± standard deviation (S.D). The significance of the differences between the means of various groups was established by one way ANOVA with a Tukey’s post hoc test using the Graphpad Prism 4 software. The p value < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Phytochemical Screening and Determination of Antioxidant Components
Phytochemical screening of MC revealed the presence of alkaloids, phenolic compounds, steroids, terpenoids, flavonoids, saponins, tannins and ascorbic acid. The ascorbic acid and total phenolic content was estimated to be 83.5 ± 9.3 mg ascorbic acid/100g of the MC and 279.2 ± 11.1 micromoles gallic acid equivalents/100g of the MC, respectively.
3.2. Effect of MC Treatment on Blood Glucose and Body Weight
Blood glucose concentration significantly increased in STZ induced diabetic mice. Administration of MC in diabetic mice significantly reduced blood glucose concentration in a dose dependant manner (Table 1). However, lycopene had no significant effect on blood glucose concentration in diabetic mice. Administration of insulin in diabetic mice maintained blood glucose in euglycemic range.
Body weight of STZ induced diabetic mice was significantly reduced compared to normal mice, while as MC and lycopene had no marked effect on body weight in diabetic mice. However, insulin treatment attenuated diabetes induced decrease in body weight in diabetic mice (Table 1).
3.3. Effect of MC on Behavioural Studies
In normal animals in hot plate test paw withdrawal latency from radiant heat was almost same (about 7.5 sec) throughout the experiment. STZ induced diabetic mice showed significant decrease in the paw withdrawal latency to thermal stimuli than normal animals indicating development of thermal hyperalgesia. However, administration of MC to diabetic animals’ significantly attenuated diabetes induced decrease in pain threshold dose dependently compared to the diabetic control. Moreover, treatment with insulin or lycopene also attenuated diabetes induced decrease in pain threshold significantly (Figure 1).
Injection of 5% formalin into the hind-paw of control animals evoked a series of flinching responses of the afflicted paw both in diabetic and normal animals. In diabetic animals number of flinches increased significantly as compared to normal animals. However, upon
Table 1. Effect of MC treatment on blood glucose levels and body weight.

Results are expressed as Mean ± S.D. (n = 10). ap < 0.05 vs NC; bp < 0.05 vs DC. NC: Normal Control; DC: Diabetic Control; MC-200: M. charantia 200 mg/kg treated diabetic animals; MC-400: M. charantia 400 mg/kg treated diabetic animals; MC-800: M. charantia 800 mg/kg treated diabetic animals; Insulin: Insulin (8 IU/Kg of porcine zinc insulin suspension and 3 IU/Kg of porcine regular insulin s.c., b.i.d.) treated diabetic animals; Lycopene: Lycopene 5 mg/kg treated diabetic animals.

Figure 1. Effect of MC on pain threshold in Hot Plate Test a = p < 0.05 vs NC; b = p < 0.05 vs DC. NC: Normal Control; DC: Diabetic Control; MC-200: M. charantia 200 mg/kg treated diabetic animals; MC-400: M. charantia 400 mg/kg treated diabetic animals; MC-800: M. charantia 800 mg/kg treated diabetic animals. Insulin: Insulin (8 IU/Kg of porcine zinc insulin suspension and 3 IU/Kg of porcine regular insulin s.c., b.i.d) treated diabetic animals; Lycopene: Lycopene 5 mg/kg treated diabetic animals.
MC treatment to diabetic animals’ noxious stimulus was significantly reduced dose dependently as reflected by decrease in number of flinches in 30 min. Moreover, administration of insulin or lycopene significantly reduced flinching response in diabetic mice (Figure 2).
The nociceptive threshold assessed by tail flick test in diabetic mice was significantly decreased compared to the normal animals. However, administration of MC in diabetic animals, significantly, attenuated diabetes induced decrease in nociceptive threshold in a dose dependent manner. Moreover, treatment with insulin or lycopene significantly attenuated diabetes induced decrease in nociceptive threshold (Figure 3).
Administration of MC, insulin or lycopene did not produce any impairment on sensory and motor functions as assessed by rota-rod test in both normal and diabetic mice (data not shown).
3.4. Effect on Oxidative-Nitrosative Stress
An increase in serum TBARS and nitrite/nitrate concentration and decrease in SOD activity was noted in STZ induced diabetic animals compared to normal animals suggesting enhanced generation of ROS and RNS (oxidative-nitrosative stress). However, administration of MC significantly attenuated dose dependently diabetes induced increase in TBARS and nitrite/nitrate concentration. Moreover, treatment with insulin or lycopene markedly attenuated diabetes induced oxidative-nitrosative stress. Moreover, diabetes induced decrease in SOD activity was also markedly restored by MC, insulin and lycopene treatment (Table 2).
4. DISCUSSION
The present study was undertaken to determine whether
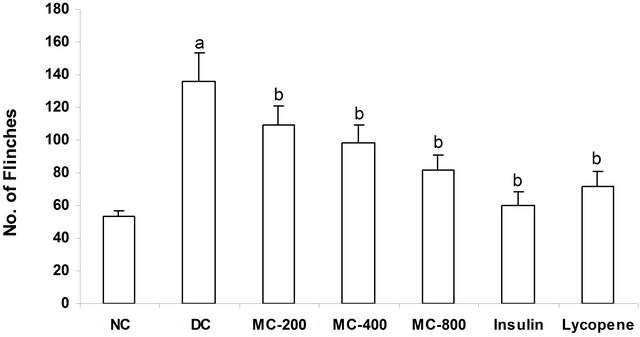
Figure 2. Effect of MC on form alin induced flinches. a = p < 0.05 vs NC; b = p < 0.05 vs DC. NC: Normal Control; DC: Diabetic Control; MC-200: M. charantia 200 mg/kg treated diabetic animals; MC-400: M. charantia 400 mg/kg treated diabetic animals; MC-800: M. charantia 800 mg/kg treated diabetic animals. Insulin: Insulin (8 IU/Kg of porcine zinc insulin suspension and 3 IU/Kg of porcine regular insulin s.c., b.i.d) treated diabetic animals; Lycopene: Lycopene 5 mg/kg treated diabetic animals.

Figure 3. Effect of MC on nociceptive threshold in Tail Flick Test. a = p < 0.05 vs NC; b = p < 0.05 vs DC. NC: Normal Control; DC: Diabetic Control; MC-200: M. charantia 200 mg/kg treated diabetic animals; MC-400: M. charantia 400 mg/kg treated diabetic animals; MC-800: M. charantia 800 mg/kg treated diabetic animals. Insulin: Insulin (8 IU/Kg of porcine zinc insulin suspension and 3 IU/Kg of porcine regular insulin s.c., b.i.d) treated diabetic animals; Lycopene: Lycopene 5 mg/kg treated diabetic animals.
Table 2. Effect of MC Treatment on Serum Nitrite/Nitrate, TBARS and SOD Concentration.

Results are expressed as Mean ± S.D. (n = 10). ap < 0.05 vs NC; bp < 0.05 vs DC. NC: Normal Control; DC: Diabetic Control; MC-200: M. charantia 200 mg/kg treated diabetic animals; MC-400: M. charantia 400 mg/kg treated diabetic animals; MC-800: M. charantia 800 mg/kg treated diabetic animals; Insulin: Insulin (8 IU/Kg of porcine zinc insulin suspension and 3 IU/Kg of porcine regular insulin s.c., b.i.d.) treated diabetic animals; Lycopene: Lycopene 5 mg/kg treated diabetic animals.
chronic administration of MC, an antidiabetic plant with antioxidant properties, can prevent the development of neuropathy in diabetic mice. We observed that diabetic mice administered MC were markedly prevented from the development of neuropathy. The effect appears to be mediated by better hyperglycemia control and reduction of oxidative-nitrosative stress.
Neuropathic pain is most common symptom associated with diabetic neuropathy and is associated with abnormalities in pain sensation reminiscent to those observed in diabetic individuals [28]. Thus, we evaluated the nociceptive response in diabetic mice to assess the development of neuropathy. Since no experimental model exactly assesses the degree of neuropathic pain, therefore, various thermal and chemical nociceptive models of pain including hot plate test, formalin test and tail flick test were employed in the present study to assess the neuropathic pain. It is reported that significant degree of hyperalgesia and allodynia develop after 3 weeks of STZ administration in rodents [29]. Therefore, animals were kept for 6 weeks after STZ administration to provide sufficient time for hyperglycemia to affect pain perception. Free radicals have been implicated in hyperglycemia induced neuropathy [30] and thus TBARS, nitrite/nitrate levels and reduced SOD activity have been estimated in the present study to evaluate the oxidativenitrosative stress. ROS produces malondialdehyde (MDA), an end product of lipid peroxidation which reacts with thiobarbituric acid (TBA) and is thus estimated as TBARS [31]. Therefore, in the present study MDA was estimated using TBARS assay to estimate the extent of ROS production. NO is an endogenous mediator of numerous physiological and pathological processes and high correlation between endogenous NO production and nitrite/nitrate (NOx) levels in plasma/serum has been established [32]. Elevated NO levels have been reported to cause neuronal damage and death [33]. Therefore, measurement of NOx levels provides a reliable and quantitative estimate of NO output in vivo. Superoxide dismutase (SOD) enzyme by catalyzing the dismutation of superoxide ions protects the biological integrity of cells and tissues against the harmful effects of superoxide free radicals. The levels of SOD diminish in diabetes, its complications as well as in oxidative stress conditions [33]. Therefore, SOD levels have been estimated to evaluate the endogeneous antioxidant capacity. Many agents affecting nociceptive response also affect motor and sensory responses; therefore, motor coordination was evaluated using rota rod test. It is reported that antioxidant activity, total phenolics and ascorbic acid content of MC are best retained by quick freezing [16], therefore, lyophilized powder obtained by continuous freeze drying of fresh juice was used in the study. Strict glycemic control and use of antioxidants has been reported to slow/ reverses the progression of diabetic neuropathy [9,10]. Therefore, insulin and lycopene were used as standard drugs to attain glycemic control and antioxidant effect, respectively.
In present study STZ induced diabetes mellitus markedly altered the pain perception. This observation supports the results of various earlier studies [34,35]. Hyperglycemia is reported to enhance ROS (mainly superoxide) by glucose overload in mitochondria [36,37]. Furthermore, hyperglycemia through the activation of NF-κB increases expression of iNOS and consequently enhances NO generation [38,39]. Superoxide anion upon interaction with nitric oxide forms the potent cytotoxin peroxynitrite, which causes protein nitration, lipid peroxidation and DNA damage, leading to toxic effects to nervous system and neuropathy. Thus, the diabetes induced neuropathy observed in the present may be due to hyperglycemia induced increased oxidative-nitrosative stress. This contention is supported by the results obtained in the present study that diabetic (hyperglycemic) mice had significantly increased the TBARS and nitrite/nitrate levels and reduced SOD activity. Moreover, increased oxidative-nitrosative stress and consequent neuropathy appear to be hyperglycemia specific because insulin induced euglycemic state in diabetic mice has markedly attenuated increase in oxidative-nitrosative stress as well as associated neuropathy.
The administration of MC to diabetic mice has been noted to prevent the development of neuropathy by improving hyperglycemia, decreasing oxidative stress and nitrite/nitrate level and restoring the SOD activity to near normal in a dose dependant fashion. The present study confirms the earlier reports of beneficial effect of MC on hyperglycemia [40,41], diabetic induced neuropathy [17] and oxidative stress [11]. Various mechanisms have been suggested to explain the antihyperglycemic effect of MC such as inhibition of glucose absorption [15], extra pancreatic effect [42], decrease in hepatic gluconeogenesis, increase in glucose oxidation [43], increase in number of beta cells [44] and insulinomimetic activity [45]. However, the present data, suggest that an insulinomimetic action, if one exists, would be minor because unlike insulin, MC treatment produced insignificant body weight gain compared to diabetic control animals.
As one can see from Table 1, MC displayed minor antihyperglycemic activity when administered to STZ-induced diabetic mice. It is highly unlikely that small (~7% - 16%) differences in blood glucose concentrations were responsible for significant differences in pain perception and other variables of neuropathy. It can be presumed that MC attenuated, to some extent; hyperglycemia induced enhanced oxidative-nitrosative stress by decreasing the generation of ROS and NO and salvaged the neuron damage. Furthermore, antioxidant components of MC may have helped to detoxify free radicals, restored the antioxidant homeostasis and thus markedly attenuated the oxidative-nitrosative stress induced neuronal damage. Therefore, it can be speculated that the observed beneficial effect of MC in preventing STZ-induced experimental neuropathy may be due to reduction of oxidative-nitrosative stress and consequent downregulation of superoxide ion and NO. This contention is supported by the results obtained in the present study that MC decreases the serum concentration of nitrite/nitrate, reduces the oxidative stress by decreasing serum TBARS and restoring the SOD enzyme activity. Moreover, the beneficial effect of MC in preventing the diabetes induced neuropathy and oxidative-nitrosative stress has been observed to be slightly superior to the effect produced by lycopene, well known antioxidant, probably due to its additional antihyperglycemic activity. This further supports the contention that antioxidant property of MC and not its antihyperglycemic action is primarily responsible for attenuation of diabetes induced neuropathy and associated nitrosative stress.
On the basis of the above discussion, it may be concluded that MC reduces the oxidative-nitrosative stress and consequently alleviates the development of diabetes induced neuropathy.
REFERENCES
- Tahrani, A.A., Askwith, T. and Stevens, M.J. (2010) Emerging drugs for diabetic neuropathy. Expert Opinion on Emerging Drugs, 15, 661-683. doi:10.1517/14728214.2010.512610
- Al-Wahbi, A.M. (2010) Impact of a diabetic foot care education program on lower limb amputation rate. Vascular Health and Risk Management, 6, 923-934. doi:10.2147/VHRM.S13569
- Ziegler, D. (2008) Treatment of diabetic neuropathy and neuropathic pain: How far have we come? Diabetes Care, 3, S255-S261.
- Tavakoli, M. and Malik, R.A. (2008) Management of painful diabetic neuropathy. Expert Opinion on Pharmacotherapy, 9, 2969-2978. doi:10.1517/14656560802498149
- Bouton, A.J.M. (2005) Opoids for painful diabetic neuropathy. Current Diabetes Reports, 5, 407-408. doi:10.1007/s11892-005-0046-8
- Coppey, L.J., Gellett, J.S., Davidson, E.P., Dunlap, J.A., Lund, D.D. and Yorek, M.A. (2001) Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes, 50, 1927-1937. doi:10.2337/diabetes.50.8.1927
- Cameron, N.E., Tuck, Z., McCabe, L. and Cotter, M.A. (2001) Effect of the hydroxyl radical scavenger, dimethylthiourea, on peripheral nerve tissue perfusion, conduction velocity and nociception in experimental diabetes. Diabetologia, 44, 1161-1169. doi:10.1007/s001250100626
- Obrosova, I.G., Mabley, J.G., Zsengeller, Z., Charniauskaya, T., Abatan, O.I., Groves, J.T. and Szabo, C. (2005) Role for nitrosative stress in diabetic neuropathy: Evidence from studies with a peroxynitrite decomposition catalyst. FASEB Journal, 19, 401-403.
- Winkler, G. and Kempler, P. (2010) Pathomechanism of diabetic neuropathy: Background of the pathogenesisoriented therapy. Orvosi Hetilap, 151, 971-981. doi:10.1556/OH.2010.28898
- Vallianou, N., Evangelopoulos, A. and Koutalas, P. (2009) Alpha-lipoic Acid and diabetic neuropathy. Review of Diabetes Studies, 6, 230-236. doi:10.1900/RDS.2009.6.230
- Malik, Z.A., Singh, M. and Sharma, P.L. (2011) Neuroprotective effect of Momordica charantia in global cerebral ischemia and reperfusion induced neuronal damage in diabetic mice. Journal of Ethnopharmacology, 133, 729-734. doi:10.1016/j.jep.2010.10.061
- Marles, R. and Farnsworth, N. (1997). Antidiabetic plants and their active constituents: An update. Phytomedicine, 2, 137-189. doi:10.1016/S0944-7113(11)80059-0
- Basch, E., Gabardi, S. and Ulbricht, C. (2003) Bitter Melon (Momordica charnatia): A review of efficacy and safety. American Journal of Health-System Pharmacy, 60, 356-359.
- Grover, J.K. and Yadav, S.P. (2004) Pharmacological actions and potential uses of Momordica charantia: A review. Journal of Ethnopharmacology, 93, 123-132. doi:10.1016/j.jep.2004.03.035
- Matsuda, H., Li, Y., Murakami, T., Matsumura, N., Yamahara, J. and Yoshikawa, M. (1998) Antidiabetic principles of natural medicines. III. Structure-related inhibittory activity andmodeof action of oleanolic acid glycosides on hypoglycemic activity. Chemical and Pharmaceutical Bulletin (Tokyo), 46, 1399-1403. doi:10.1248/cpb.46.1399
- Myojin, C., Enami, N., Nagata, A., Yamaguchi, T., Takamura, H. and Matoba, T. (2008) Changes in the radical-scavenging activity of bitter gourd (Momordica charantia L.) during freezing and frozen storage with or without blanching. Journal of Food Science, 73, C546- C550. doi:10.1111/j.1750-3841.2008.00886.x
- Grover, J.K., Rathi, S.S. and Vats, V.V. (2002) Amelieration of experimental diabetic neuropathy and gastropathy in rats following oral administration of plant (Eugenia jambolana, Mucurana puriens and Tinospora cordifolia) extracts. Indian Journal of Experimental Biology, 40, 273-276.
- Evans, W.C. (1989) Trease and Evans’ pharmacognosy, 13th Edition, Bailliere Tindall, London.
- Rerup, C. and Tarding, F. (1969) Streptozotocinand alloxan-diabetes in mice. European Journal of Pharmacology, 7, 89-96. doi:10.1016/0014-2999(69)90169-1
- Eddy, N.B. and Leimbach, D.J., (1953) Synthetic analgesics. II. Dithienylbutenyland dithienylbutylamines. Journal of Pharmacology and Experimental Therapeutics, 107, 385-393.
- Tjolsen, A., Berge, O.G., Hunskaar, S., Rosland, J.H. and Hole, K. (1992) The formalin test: An evaluation of the method. Pain, 51, 5-17. doi:10.1016/0304-3959(92)90003-T
- D’Armour, W.L. and Smith, D.L. (1941) A method for determining loss of pain sensation. Journal of Pharmacology and Experimental Therapeutics, 72, 74-79.
- Dunham, N.W. and Miya, T.S. (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. Journal of American Pharmaceitocal Association. American Pharmaceutical Association, 46, 208-210. doi:10.1002/jps.3030460322
- Sastry, K.V., Moudgal, R.P., Mohan, J., Tyagi, J.S. and Rao, G.S. (2002) Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Analytical Biochemistry, 306, 79-82. doi:10.1006/abio.2002.5676
- Ma, F.X., Liu, L.Y. and Xiong, X.M. (2003) Protective effects of lovastatin on vascular endothelium injured by low density lipoprotein. Acta Pharmacologica Sinica, 24, 1027-1032.
- Misra, H.P. and Fridovich, I. (1971) The generation of superoxide radical during the autoxidation of ferredoxin. Journal of Biological Chemistry, 246, 6886-6890.
- Jewett, S.L. and Rocklin, A.M. (1993) Variation in one unit of activity with oxidation rate of organic substrate in indirect superoxide dismutase assays. Analytical Biochemistry, 212, 555-559. doi:10.1006/abio.1993.1368
- Obrosova, I.G., Mabley, J.G., Zsengeller, Z., Charniauskaya, T., Abatan, O.I., Groves, J.T. and Szabo, C. (2005) Role for nitrosative stress in diabetic neuropathy: Evidence from studies with a peroxynitrite decomposition catalyst. FASEB Journal, 19, 401-403.
- Grover, V.S., Sharma, A. and Singh, M. (2000) Role of nitic oxide in diabetes induced attenuation of antinociceptive effect of morphine in mice. European Journal of Pharmacology, 399, 161-164. doi:10.1016/S0014-2999(00)00343-5
- Pop-Busui, R., Sima, A. and Stevens, M. (2006) Diabetic neuropathy and oxidative stress. Diabetes Metabolism Research and Reviews, 22, 257-273. doi:10.1002/dmrr.625
- Dib, M., Garrel, C., Favier, A., Robin, V. and Desrualla, C. (2002) Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? Journal of Neuroscience, 249, 367-374.
- Marletta, M.A., Yoon, P.S., Iyenger, R., Leaf, C.D. and Wishnok, J.S. (1988) Macrophage oxidation of L-arginine to nitrite and nitrate: Nitric oxide is an intermediate. Biochemistry, 27, 8706-8711. doi:10.1021/bi00424a003
- Aquilano, K. Baldelli, S. Cardaci, S., Rotilio, G. and Ciriolo, M.R. (2011) Nitric oxide is the primary mediator of cytotoxicity induced by GSH depletion in neuronal cells. Journal of cell Science, 124, 1043-1054. doi:10.1242/jcs.077149
- Forman, L.J., Estilow, S., Lewis, M. and Vasilenko, P. (1986) Streptozocin diabetes alters immunoreactive betaendorphin levels and pain perception after 8 wk in female rats. Diabetes, 35, 1309-1313. doi:10.2337/diabetes.35.12.1309
- Ohsawa, M. and Kamei, J. (1999) Possible involvement of spinal protein kinase C in thermal allodynia and hyperalgesia in diabetic mice. European Journal of Pharmacology, 372, 221-228. doi:10.1016/S0014-2999(99)00228-9
- Wolff, S.P. (1987) The potential role of oxidative stress in diabetes and its complications: Novel Implication for theory and therapy. In: Crabba, M.J.C., Ed., Diabetic Complication: Scientific and Clinical Aspects, ChurchillLivingstone, New York, 167-200.
- Eriksson, U.J. and Berg, L.A.H. (1993) Diabetes and embryonic malformation role of substrate-induced free oxygen radical production for dysmorphogenesis in cultured rat embryos. Diabetes, 42, 411-419. doi:10.2337/diabetes.42.3.411
- Calles-Escandon, J. and Cipolla, M. (2001) Diabetes and endothelial dysfunction: A clinical perspective. Endocrine Reviews, 22, 36-52. doi:10.1210/er.22.1.36
- Cosentino, F., Hishikawa, K., Katusic, Z.S. and Luscher, T.F. (1997) High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation, 96, 25-28. doi:10.1161/01.CIR.96.1.25
- Grover, J.K., Vats, V., Rathi, S.S. and Dawar, R. (2001) Traditional Indian anti-diabetic attenuate progression of renal damage in streptozotocin induced diabetic mice. Journal of Ethnopharmacology, 76, 233-238. doi:10.1016/S0378-8741(01)00246-X
- Srivastava, Y., VenkataKrishna-bhatt, H., Verma, Y. and Prem, S. (1987) Retardation of retinopathy by Momordica charantia L. (Bitter gourd) fruit extract in alloxan diabetic rats. Indian Journal of Experimental Biology, 25, 571-572.
- Welihinda, J., Karunanayake, E.H., Sheriff, M.H. and Jayasinghe, K.S. (1986) Effect of Momordica charantia of the glucose tolerance in maturity onset diabetes. Journal of Ethnopharmacology, 17, 277-282. doi:10.1016/0378-8741(86)90116-9
- Shibib, B.A., Khan, L.A. and Rahman, R. (1993) Hypoglycemic activity of Coccinia indica and Momordica charantia in diabetic rats: Depression of hepatic gluconeogenesis enzymes glucose-6-phosphatase and fructose 1,6-bisphosphatase and elevation of both liver and red cell shunt enzyme glucose-6-phosphate dehydrogenase. Biochemical Journal, 292, 267-270.
- Ahmed, I., Adeghate, E., Sharma, A.K., Pallot, D.J. and Singh, A. (1998) Effect of Momordica charantia fruit juice on islet morphology in the pancreas of the streptozotocin diabetic rat. Diabetes Research and Clinical Practice, 40, 145-151. doi:10.1016/S0168-8227(98)00022-9
- Rathi, S.S., Grover, J.K. and Vats, S. (2002) The effect of Momordica charantia and Mucuna puriens in experimental diabetes and their effect on key metabolic enzymes involved in carbohydrate metabolism. Phytotherapy Research, 16, 236-243. doi:10.1002/ptr.842
NOTES
*Corresponding author.

