World Journal of Cardiovascular Diseases
Vol.3 No.4A(2013), Article ID:34776,12 pages DOI:10.4236/wjcd.2013.34A006
Re-engineering in OPCAB—A Vettath’s perspective
![]()
Department of Cardiothoracic and Vascular Surgery, International Center of Excellence in OPCAB Surgery, Malabar Institute of Medical Sciences, Kozhikode, India
Email: *murali.vettath@gmail.com
Copyright © 2013 Murali P. Vettath et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 10 May 2013; revised 8 June 2013; accepted 17 June 2013
Keywords: CABG (Coronary Artery Bypass Grafting); OPCAB (Off Pump Coronary Artery Bypass); ONCAB (On Pump Coronary Artery Disease); Diffuse Coronary Artery Disease; IABP (Intra-Aortic Balloon Pump); CPB (Cardiopulmonary Bypass)
ABSTRACT
OPCAB (off pump coronary artery bypass) has become a preferred technique of coronary revascularization in India, and more so in the East. This technique was restarted by Buffalo and Bennetti who had published their results in 1985. Since then, there has been a great enthusiasm among coronary surgeons to develop and standardize this technique of CABG (coronary artery bypass grafting). In the late nineties, nearly all the coronary centers in India started performing this technique. But, by the early 2000, only a few surgeons continued this practice. Only those who could perform this OPCAB technique in nearly 100% of their patients continued this and the rest of them returned back to the conventional on pump CABG. To attain this result, we had to re-engineer our technique of anesthesia, surgical technique, stabilization, and positioning of the heart to enable us to perform OPCAB in all patients who needed CABG. We have analyzed our last 3000 patients operated by the same surgeon (Dr MPV), in the same center with the same team. As OPCAB was the only procedure performed for coronary revascularization, we have compared our first 1000 patients, with the second 2000 patients that underwent the procedure. Our technique and our results are presented.
1. INTRODUCTION
In 1981, Enio Buffolo [1] from Brazil and Benneti [2] from Argentina had started experimenting on this technique of direct Myocardial revascularisation. Both of them published their series simultaneously, around 1985 which rekindled the idea of OPCAB in the western world. It was probably the idea of mini-invasive direct coronary artery bypass graft (MIDCABG), introduced in the mid- 1990s by Benneti [2,3], that called attention to the possibility and advantages of not using CPB. Calafiore’s [4,5] publications re-enforced the advantages of the LAST (left anterior small thoracotomy) operation. The LIMA stitch was acclaimed as an extraordinary step in the development of off-pump coronary surgery, which allowed grafting of posterior branches of the coronary arteries. The introduction of stabilizers in the mid-1990s further facilitated the procedure [6]. Dr. Eric Jansen, cardiac surgeon from Utrecht, Netherland, was one of those surgeons in the mid-nineties, who was probably the man who had made the word—Octopus—so very popular in the rest of the world. The article published in 1991 by Benetti [7] in Chest, gave confidence to the Cardiac surgeons around the world to perform OPCAB in all anterior vessels. The circumflex territory was still a danger zone for most surgeons to perform a safe coronary anastomosis. Though LIMA stitch was used quite often, it was the availability of the positioners that that made the process of verticalisation of the heart more comfortable.
By the end of 1990, most of the surgeons in India and in the far east had been performing OPCAB in 90% of their coronary artery patients, but by early 2000, there was a sharp decline in the numbers, as most of the surgeons did not find the comfort zone in OPCAB surgery, and that their patency rates were being questioned. In the late 1990s, the visibility of the coronary anastomosis was again a doubtful proposition, and also converting and going on to the pump became a recipe for disaster. Then came the comparative trials of OPCAB and ONCAB, which obviously brought in results which showed that both the techniques produce nearly the same results, and that the patency was a question of concern [8-10]. The Rooby trial showed that, at 1 year of follow-up, patients in the off-pump group had worse composite outcomes and poorer graft patency than did patients in the on-pump group [11]. The surgeons have now come to a conclusion that OPCAB is good in experienced hands and the results in this group of people have been outstanding.
The appeal of avoiding cardiopulmonary pass with its direct and indirect physiological insult, the prospect of improved clinical outcomes, and the favorable economic impact gives OPCAB the potential of preference that may mark the dawn of a new era in our search for the optimal surgical strategy for the treatment of coronary artery disease.
OPCAB has been performed in many different ways. It’s like different ways of skinning a cat. Ultimately, the gold standard of a perfect patent coronary anastomosis remains the corner stone of a good surgeon and a good operation. It is to be emphasized here that coronary artery bypass surgery has a come a long way from performing them off pump, then on pump and now going back to off pump. But the most important point, one has to bear in mind is that, the surgeon has to do what he is most comfortable with and by which he would be able to deliver the best result.
The topic of re-engineering in OPCAB came up, because, there was an engineering that was done during the early phase of OPCAB by the great pioneers of this procedure. But what happened along the way was that this procedure was not reproducible by lesser mortals like us and hence we had to re-engineer this procedure to suit us.
2. MYOCARDIAL PRESERVATION
Adequate myocardial preservation is crucial to perform CABG. Preoperative resuscitation of ischemic myocardium enables recruitment of hibernating myocardium and forms an important component of any myocardial protection strategy. The intraoperative strategy varies (within physiological boundaries) as much from patient to patient as it is from surgeon to surgeon, to the extent that a good clinical outcome becomes the ultimate determinant of the optimal strategy. Even with the same surgeon, the strategy is adapted to the patient and clinical scenario that a prescriptive regimen is not standard. The objective of intraoperative myocardial preservation is to enable efficient myocardial energy management by reducing cardiac metabolic demands on the one hand, while improving myocardial oxygen supply and utilization on the other [12].
In on-pump CABG, cardioplegia or cross-clamp fibrillation are conventional methods of intraoperative myocardial protection. Cardioplegia favorably affects myocardial energy metabolism but results in the alteration of both the intraand extra-cellular milieu and this, together with CPB (cardiopulmonary bypass), can precipitate changes in cardiac performance postoperatively [13]. Cross-clamp fibrillation can increase the endocarpdial viability ratio and lead to similar changes in cardiac function. In both strategies of myocardial protection, a period of global myocardial ischemia is followed by reperfusion with oxygen-rich blood predisposing to reperfusion injury which manifests as myocardial stunning and arrhythmias in the early postoperative period.
Since deliberate induction of global ischemia is unnecessary in OPCAB, it is logical to suppose that iatrogenic biochemical injury to the myocardium would not occur. More so, the blunted inflammatory response with avoidance of CPB is characterized by low production of IL-8 which is involved in myocardial injury. In fact, Atkins, et al. first suggested that OPCAB preserved cardiac function in 1984 [14]. In different prospective randomized studies, Ascione [15], Penttilä [16], Van Dijk [17], Czerny [18], Bennetts [19], and Masuda [20], and their collaborators reported minimal change in the biochemical markers of myocardial injury (troponin T and/or creatinine kinase-MB isoenzyme), and in some cases, better myocardial function after OPCAB compared to on-pump CABG. Changes in myocardial metabolism indicative of oxidative stress due to local ischemia when the target coronary artery is occluded to enable visualizetion for distal anastomoses have been reported in OPCAB [21]. Compared to on-pump CABG, OPCAB is associated with better myocardial energy preservation, less oxidative stress and minimal myocardial damage [16]. However, emerging evidence suggests that intraoperative myocardial protection in OPCAB can provide an added advantage [22,23]. Vassiliades, et al. [24] compared active coronary perfusion using a perfusion pump, with passive perfusion by a cannula connected from the aorta to the graft, and no coronary perfusion, after the distal anastomosis in a randomized clinical trial. They found lower troponin I levels with active and passive coronary perfusion, but cardiac performance was better with active coronary perfusion. The use of intra-coronary shunt during OPCAB has also been shown to preclude left ventricular dysfunction [25].
Reperfusion injury can occur from regional ischemia due to a combination of underlying coronary obstructive pathology, stabilization and anastomotic techniques, compounded by episodes of hypotension which precede revascularization. The precarious normoxic and normothermic passive coronary perfusion may be insufficient to protect against myocardial damage in such clinical scenarios.
The concept of myocardial protection in OPCAB is less tedious. In most cases passive coronary perfusion with intra-coronary shunts will suffice, but in the presence of heightened cardiac risk such as recent acute myocardial ischemia or infarction, and severely impaired left ventricular function active coronary perfusion is advantageous especially in multi-vessel revascularization.
The role of intra-aortic balloon pump (IABP) in patients with ischemic myocardium and in low ejection fraction would be discussed later in the article. As of today, apart from using intra coronary shunt, the use of aortocoronary shunt in some instances has been a disposable worth remembering.
Hemodynamic instability has been the major concern in performing OPCAB even today. Though we have been able to master the technique of positioning the heart by the use of various technique and devices, this still remains a major concern. Exposure of the coronary artery target sites requires the heart to be lifted, rotated, dislocated and displaced producing a distortion of cardiac geometry and consequently hemodynamic fluctuations frequently occur. As a result, the early reports of OPCAB described single or double grafts limited to anterior target sites. The corrective measures for these hemodynamic changes include volume loading, trendelenberg positioning, and displacement of the heart into the opened right pleura, use of inotropes, vasopressors, vasodilators, intra-aortic balloon pump, and right heart circulatory support [9,26].
What we had re-engineered over the last ten years is to avoid the Trendelenberg position (Figure 1). We practice anti-Trendelenberg position of the patient (where the patient lies on the table with the head end up). This is very useful in patients with ischemia, where the pulmonary artery (PA) pressure is high. This maneuver reduces the PA pressure, and there by reduces the left ventricular end diastolic pressure (LVEDP). This is exactly what the patient would do when he develops chest pain in his room. He sits up and tries to catch his breath. That is what we help him do in the theater as he is anaesthetized. If the CVP (central venous pressure) is low and the right ventricle looks empty, then we give a fluid challenge to improve his preload. But we always try to avoid the Trendelenberg position.

Figure 1. Showing the table in anti-trendelenberg position.
In the late nineties, the principle of OPCAB was to perform the coronary anastomosis at a heart rate of less than 60 per minute. Hence, the patients coming for surgery used to be well beta blocked, so that it would be easy to perform the anastomosis. But, most of these patients ended up having inotropes and vasoconstrictors to maintain hemodynamics. Ischemic patients when given either of them, they develop further ischemia and become bad. What we had re-engineered here was to increase the heart rate alone. To attain that, we use intermittent boluses of Injection Atropine (0.6 mgs per milliliter), which is only a Chronotropic agent and not an Inotrope. Thus, avoiding unnecessary strain on the myocardium, we aim at a heart rate above 100 per minute and this keeps the heart briskly contracting and avoiding the usual hemodynamic collapse seen in the early days. We do not use vasopressors or vasodilators in any of our patients undergoing OPCAB. The only drug the patient would be given intermittently is the Atropine injection. We use Glycerol tri-nitrate (GTN) in our patients after the distal anastomosis and that too, only as an antihypertensive.
Mueller, et al. found no change in the haemodynamics during exposure of the posterior and anterior wall arteries, and only marginal change for the lateral wall artery with a “no compression” technique [27]. It has been suggested that the use of left ventricular apical suction device for cardiac positioning evokes less hemodynamic instability compared with pericardial retraction sutures [28].
The technique which we have developed is to cut down the pericardium on the right side, down to the inferior vena cava, and to leave the right pleura opened. We routinely open the left pleura to help in the mammary dissection. Hence, in all our patients we have all the three chambers—the mediastinum and both the pleura cavity, remaining in continuity to each other. We routinely use the positioner for lifting the apex of the heart. The suction pressure used is not more than 100 mm of Hg. The positioner is always used in an off apex position, so as to avoid sucking the left anterior descending (LAD) coronary artery. We use this low pressure (unlike what is recommended by the manufacturer) to avoid damage to the myocardium. We also watch the suction tube, to see if any blood is being sucked. In such cases, the positioner is removed and repositioned in a different place. In case of grafting of the circumflex and the posterior descending artery (PDA), the heart is lifted and verticalized and not tilted to the right side. This lifting of the apex increases the left and the right ventricular volume. And in case the PDA is being grafted, then we tilt the head end down, so as to increase the visibility of the PDA. This probably would be the only time when the Trendelenberg position is used. By this time the LAD is already perfused, and this has relieved the ischemia. If the visibility of the circumflex is bad, then the table is dropped down to its maximum and the positioner moved to right a bit. With this maneuver, even the atrioventricular groove could be visualized.
After positioning the heart, the next important issue to be tackled was to achieve an excellent distal anastomosis. This is achieved by using stabilizers.
3. QUALITY OF DISTAL ANASTAMOSIS
Performing vascular anastomoses on small arteries on a beating heart can be a daunting and frustrating adventure, and so far, no available method of target vessel stabilization could achieve a steady bloodless field comparable to a cardioplegically arrested heart. This was a major concern with OPCAB. The beating heart with a bloody operating field poses a major challenge to delicate tissue handling, and casts a shadow of uncertainty about the quality of the distal anastomosis. However, with the application of effective target vessel stabilization, and efficient visualisation systems the early and mid-term patency of OPCAB has been encouraging.
The stabilizers that we use are the suction stabilizers. The position of the stabilizer is very important to achieve a very stable anastomotic site to perform a good coronary anastomosis. We have tried all types of suction stabilizers, from the Medtronic-Octopus II, III and the Octopus IV. We are now using the Maquet, which was the previous Guidant-Acrobat stabilizer. The re-engineering in this is the pressure used for suction of these stabilizers. We use 100 mm of Hg on these stabilizers. The stabilizer is positioned according to the convenience of the surgeon. The position of our stabilizer is shown in the photographs (Figure 2). We do not use any suction while stabilizing the lateral wall. The heart is allowed to fall on the stabilizer.
In order to achieve a stable bloodless field while ac-

Figure 2. Lad with shunt.
cessing the coronary artery, we use the following technique. After stabilizing the coronary artery by using the acrobat stabilizer, we use a 5.0 polypropylene suture to run around the proximal part of the coronary artery, proximal to where the arteriotomy is planned. The two ends of the 5.0 suture are suspended using rubber shod. They are tightened just before the coronary arteriotomy is made. This is made using the bevel of an 18 gauge needle on a 2 ml syringe. After the nick is made on the coronary artery, the forward or the backward cutting scissor is used to open the coronary artery. A Castroviejo scissors is used to open the arteriotomy. The arteriotomy is usually about one centimeter long. Then the intracoronary shunt is inserted according to the size of the coronary artery. The shunt is to be deaired after inserting the proximal end first, then the snare is released and the then the distal end is inserted. Then the anastomosis of the coronary is performed using the conduits as preferred. We use shunts in nearly all the distal anastomosis (Figures 3 and 4).

Figure 3. Showing distal anastamosis-lateral wall.

Figure 4. Showing distal anastamosis-post wall.
4. INCOMPLETE MYOCARDIAL REVASCULARISATION
Early reports of OPCAB in the literature were uniformly consistent in the low number of grafts per patient. The selection of patients with mainly single-vessel disease may, in part, explain this finding. But the persistence of lower average number of grafts in later comparative studies [29,30] places OPCAB in a contentious position which detracts from its potential benefits. In their retrospective study, Gundry and colleagues reported a significantly lower mean number of grafts, and a two-fold increase in cardiac re-intervention rate during a 7-year period with off-pump performed without cardiac stabilization, compared to on-pump CABG. This finding has been corroborated by other reports, which exemplifies incomplete revascularization with OPCAB. Effective cardiac retraction, stabilization and visualisation with patient positioning enables grafting of all graftable target vessels, making complete myocardial revascularisation (CMR) attainable in OPCAB [31,32], and this has been demonstrated in a recent prospective randomised study [33]. However, incomplete myocardial revascularisation with OPCAB is still reported in retrospective studies. Technical difficulties due to small caliber of target vessels or their intramyocardial course, poor exposure of target sites, precarious intraoperative haemodynamic state, electrophysiological instability and inexperience of the surgeon are some of the reasons for incomplete myocardial revascularisation.
Today, we have no contraindications for OPCAB, the intramyocardial coronary arteries, small coronary arteries and diffuse coronary arteries [34,35], have been a thing of the past. Any patient who needs to undergo CABG could have his coronary artery bypass surgery done using the OPCAB technique (Figures 5 and 6).
5. HIGH RISK SURGICAL PATIENTS
OPCAB has been demonstrated to offer prognostic advantage over on-pump CABG in patients with exaggerated surgical risk from complicated coronary artery disease and/or debilitating co-morbidities [36-39]. More importantly, the preoperative optimization of high risk patients plays a crucial role in determining the clinical outcome for both methods of myocardial revascularization.
Acute myocardial infarction and depressed left ventricular function constitute a high surgical risk with onpump CABG, because the myocardial damaging effects of CPB and the often cumbersome and, inefficient intraoperative myocardial protection do not prevent immediate postoperative cardiac dysfunction [12,40,41]. OPCAB achieves comparatively better outcomes in patients who have myocardial revascularization soon after recent
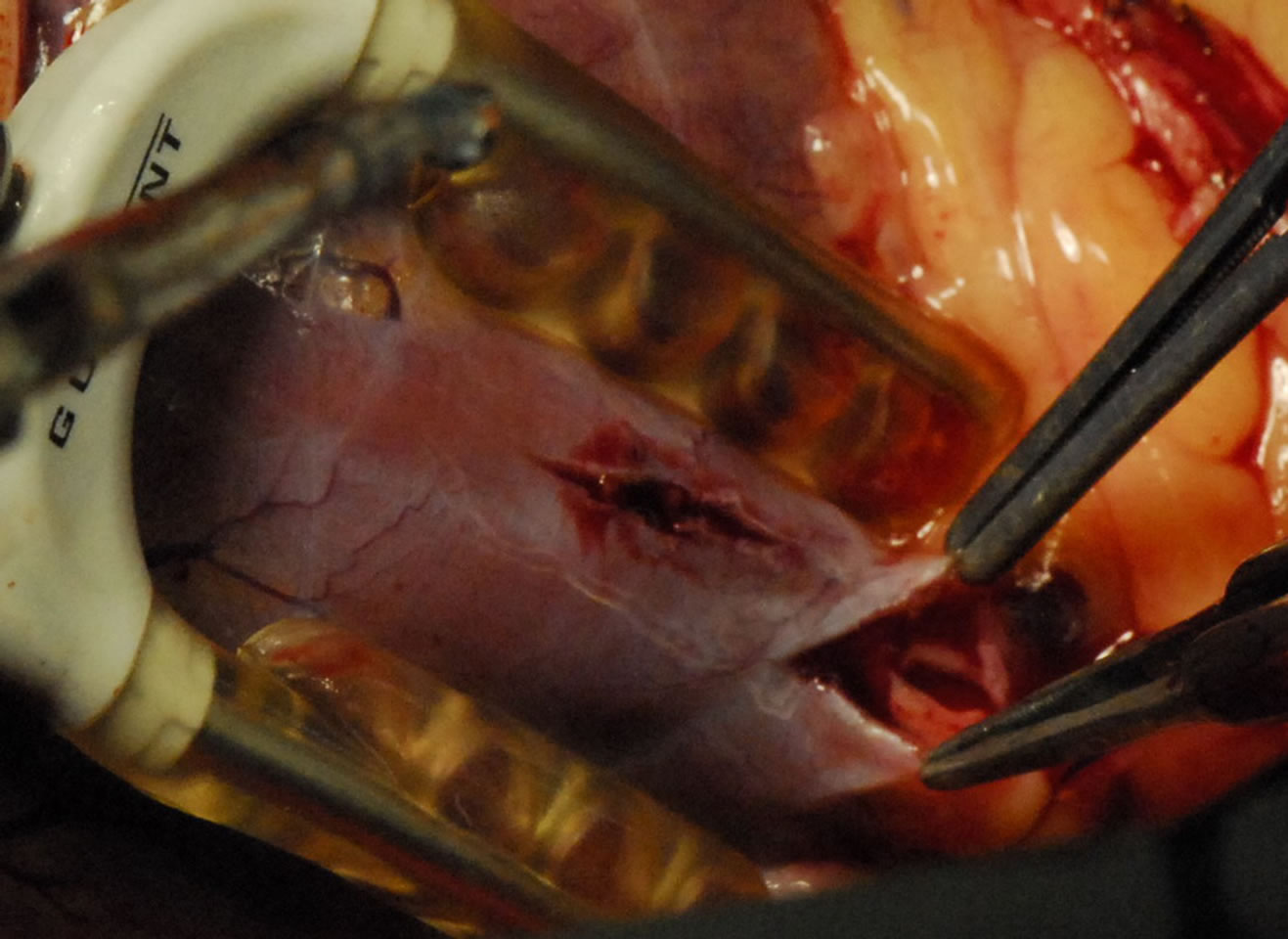
Figure 5. Showing Intramuscular coronary.

Figure 6. Showing grafted Intramuscular coronary artery.
AMI (acute myocardial infarction) [42]. Mohr, et al. [43] reported a mortality of 1.7% with 1- and 5-year actuarial survival rates of 94.7% and 82.3%, respectively, in a series of 57 patients in which 56% had emergency surgery within 48 hours of acute myocardial infarction and some were in cardiogenic shock. OPCAB decreases the operative risk in the presence of impaired left ventricular function [44].
Preoperative renal impairment is an independent predictor of poor prognosis after on-pump CABG [45]. OPCAB preserves renal function better than on-pump CABG [46], and available evidence favors the preferential use of OPCAB for patients with chronic renal failure, for a better early clinical outcome.
Patients with coexisting chronic obstructive airway disease derive better early clinical benefit from CABG performed without CPB compared with on-pump surgery [47], although in low-risk patients, OPCAB induces impairment of the mechanics of the respiratory system, lung and chest wall similar to on-pump CABG [48].
Elderly patients are considered high risk surgical patients because of their reduced functional capacity and the presence of co-morbidities. Correspondingly, the outcome of on-pump CABG in this group is characterized by increased morbidity and mortality [49,50]. Interestingly, OPCAB has been shown to improve the clinical outcome in this growing population of surgical patients [51-54]. Specifically, the incidence of stroke, perioperative myocardial infarction, and duration of mechanical ventilation, blood transfusion, length of intensive care and hospital stay and mortality are decreased.
6. VETTATH’S ANASTAMOTIC OBTURATOR
In patients with diffusely diseased coronary arteries and in patients with diseased aortas, OPCAB has remained a life saver. We had developed the Vettaths anastomotic obturator (VAO) [55,56], which is an aortic anastomosis enabling device (Figure 7). This allows the surgeon to avoid the side clamp on the aorta, when a no touch technique is required in cases of diseased aorta. In patients with plaquey aortas, where a saphenous vein top end is to be connected, this could be used to make an anastomosis on a non plaquey zone in the aorta. The technique is to identify a soft spot on the aorta, and make two purse string sutures with 3.0 polypropylene around the intended zone of anastamosis. The purse strings are about a centimeter in diameter. A stab wound is made using a no.11 blade knife, and an aortic punch is used to make a punch hole on the aorta. The VAO is then inserted into the hole and one of the 3.0 purse strings is used to snare the bleeding around the VAO if bleeding persists. The aortic systolic pressure should be maintained at around 100 mm of Hg. The advantage is that this allows the surgeon to perform a hand sewn anastomosis on the vein graft (Figures 8-10). This obturator is not like the devices

Figure 7. Showing VAO.

Figure 8. Showing-VAO TOP end anastamosis in progress and everted aortic rim.

Figure 9. Showing-TOP end after using VAO.
that are available in the market, like the Heartstring and the Enclose device. This is like an instrument and is made of steel and can be reused and could help in avoiding a stroke in elderly patients. We have performed more than 500 top end anastomosis using this device and it is a good one to have in the armamentarium of a cardiac surgeon. This is also a good tool to be used in redo CABG, when a top end anastomosis could be made on the hood of the old vein graft. The extended use of VAO is shown in (Figure 11).

Figure 10. Showing different stages of anastamosis using VAO.

Figure 11. Showing extended use of VAO.
7. VETTATH’S TECHNIQUE OF LONG MAMMARY PATCH
We had devised our own technique of performing OPCAB for patients with diffusely diseased coronary arteries (Figure 12) The Vettath’s technique of long mammary patch without endarterectomy has been published before in the HSF (Heart surgery forum) [37].
A single stabilizer is used if the arteriotomy is <4 cm. If it exceeds >4 cm, two stabilizers (one facing each other) (Figure 13) are used for coronary stabilization. The arteriotomy extends distally to reach the normal lumen of LAD. Proximal extent of arteriotomy is kept just short of the most severe proximal lesion to avoid competitive flow from native LAD. Distal coronary perfusion during anastomosis is maintained using conventional intracoronary shunts (Clearview, Medtronic Inc, Minneapolis, MN, USA). If the arteriotomy exceeds 3 - 4 cm, cut ends of aortocoronary shunts (Quickflow, Medtronic Inc, Minneapolis, MN, USA) are used. These may be tailored to use in arteriotomies upto 6 - 7 cm. In this

Figure 12. Showing diffusely diseased coronary arteries.

Figure 13. Showing long mammary patch anastamosis.
technique, hither to undescribed (Vettath’s modification of aortocoronary shunts), the distal perfusion tips of aortocoronary shunts are cut and inserted into the coronary artery. The bulb is inserted into the end from where the blood flows (i.e., into distal coronary lumen if the flow is retrograde and vice-verse). If the shunt does not sit inside the coronary (or it bowstrings), it is tacked down with a tacking suture taken in the midpoint of arteriotomy. This tacking suture is taken out at the end of anastomosis, along with the shunt. We use 7.0 polypropylene for this tacking suture. Occasionally, when native coronary flow is negligible and/or coronary lumen is <1 mm, the LAD is snared proximally with circumferential suture and LIMA to LAD anastomose was done. LIMA is slit to match the coronary arteriotomy and LIMA to LAD anastomosis is performed using 7.0 polypropylene. The plaques are excluded from the lumen of the reconstructed LAD. Diagonals and perforators are included in the new lumen. Posterior 25% of reconstructed coronary artery is formed by native coronary artery and anterior 75% by LIMA. Approximately 10 minutes were taken to construct a 2 cm patch and an additional 5 minute per each added centimeter of patch were taken for anastomosis.
The advantage of this technique is that the intima is left intact and no injury is made on it. The avoidance of endarterectomy is a definite reason for the patency in our study. We also do not add any anticoagulants or anything other medications, than those used for the normal CABG patients. Also that inspite of our long anastamosis, patients remain quite stable all along the anastamotic time. This technique of long patch has also been described by Takanashi [57].
8. ROLE OF IABP IN OPCAB
The use of intraaortic balloon pump (IABP) either preoperatively or intraoperative, to reduce operative risk and to facilitate posterior vessel OPCAB has been well documented. IABP has been useful in high-risk patients with left main coronary artery disease (>75% stenosis), intractable resting angina, post infarction angina, left ventricular dysfunction (ejection fraction <35%), or unstable angina.
Preoperative IABP counter pulsation has been shown to have better outcomes compared with perioperative or postoperative insertion in critical patients, and off-pump surgical procedures have been advocated to reduce mortality in high-risk patients.
In patients with high risk factors, higher mortality and morbidity rates have been demonstrated in spite of massive pharmacologic support combined with postoperative IABP support. IABP therapy results in a more favorable myocardial blood supply, increased stroke volume and cardiac output through augmentation of the diastolic pressure, and afterload reduction [58,59]. Intraoperative or postoperative IABP insertion has been reported to be associated with higher operative mortality rate and device-related complication rate, as compared with preoperative use of IABP.
In our center, any patient who has a hemodynamic compromise or has an inclination to crash, gets an IABP inserted sheathless. (7 or 8 French). We had the IABP inserted in the early days when we had the patient included in one of the high risk group, like left main coronary artery disease (>75% stenosis), intractable resting angina, ST depression more than 2.5 mm, post-infarction angina, left ventricular dysfunction (ejection fraction <35%), or unstable angina. We had noticed that the use of IABP was not high in the left main disease group and low ejection fraction group, but it was high in patients with ongoing ischemia.
Hence we re-engineered our use of IABP such that every patient undergoing OPCAB gets a femoral arterial line and this is used for monitoring, along with the radial arterial line. When a patient becomes ischemic during the process of lifting the heart and while positioning for lateral wall grafting, then he is repositioned and a sheathless IABP is inserted. This is then used till the distal anastamosis is over. Once the anastomosis is complete and the heart repositioned for the top end anastamosis, then the IABP is kept on standby mode. After the top end anastamosis is over, the heparin is reversed. Once the reversal is over and the patient remains hemodynamically stable, we remove the IABP on the table, after inserting another femoral arterial line in the other groin. This technique has been very useful in avoiding conversions in our series. We have been following these patients for the past five years. In fact this technique has been sent for publication. We have not had to reintroduce any IABP in the ICU any of these patients over the last five years.
9. RESULTS
We had audited our last 3000 OPCAB performed by a single surgeon (Dr MPV) in a single center over the last 10 years. The results have been elucidated in the Table 1.
The first 1000 patients were compared with the second 2000 consecutive OPCABs (Table 2). We had noticed a higher conversion rate (to the heart lung machine) in the first 1000 compared the second 2000. Probably that was because of our learning curve, and also that the use of IABP was low in the initial years. And now that we had

Table 1. Showing OPCAB results over 10 years.

Table 2. Showing OPCAB Audit fraction numbers.
standardized our technique over the last 5 years, our results have improved (Table 2). With our modification of the use of IABP in OPCAB, our conversion rates to the heart lung machine has sharply declined. We have been able to demonstrate that the IABP could be used for maintaining stable hemodynamics while performing distal anastamosis in ischemic patients. After the grafting is complete, the IABP could be removed in the theater itself. Unlike the normal dictum of—once the IABP was inserted, leave it for 24 to 48 hours. The only conversion that happened in the last 2000 patients was when the patient developed intractable arrhythmia. Here the IABP could not help maintain hemodynamics, hence had to go on the heart lung machine to save the patient.
Our use of inotropes is now negligible in the present series. The number of grafts has increased marginally. We had noticed that the patients developing perioperative myocardial infarction, post-operative renal failure and stroke had also decreased—as noted in the table. With the re-engineering of our OPCAB technique, we have been able to operate on patients with chronic renal failure and with renal transplant with good results.
10. CONCLUSIONS
The advances that have been made in the surgical management of coronary artery disease has placed us in a position to judge the outcome of our management techniques not only by morbidity and mortality incurred, but also by the potential of our treatment modality to cause harm to the patient This is why OPCAB has generated renewed, widespread and sustained interest. The resurgence of OPCAB has also ignited a keen enthusiasm in the refinement of CPB techniques and the management of on-pump CABG patients. In most practices, OPCAB is paradoxically dependent on, and guaranteed by the presence of the CPB machine. We would like to stress here that OPCAB is not for everyone. It is definitely not for the faint hearted surgeon. It needs a team with a MINDSET. And the team has to gear itself from being able to perform CABG on full cardiopulmonary bypass, with cross clamp and cardioplegia, to performing CABG on pump with a beating heart, and then going on to just cannulating the aorta, and then stabilising the heart and performing OPCAB to doing a full OPCAB. This should be a slow transition than a sudden change. Then the results would be good. There has been numerous articles comparing OPCAB with ONCAB, but in our opinion, a surgeon performing OPCAB would not have to perform ONCAB, whatever the coronary anatomy is, if he sets his mind to it. Hence, as we do not have an on pumps series, we had to compare our early 1000 patients with our second 2000 patients to study and audit our work.
In our last 10 years of OPCAB experience and over 3000 OPCABs, we were able to perform the last 2000 OPCABs with only one conversion to the heart lung machine. (That was when patient developed intractable arrhythmia).
Hence in our opinion, intractable arrhythmia is the only reason for conversion. The 30-day mortality in the first one thousand patients has been 0.8% and in the second thousand is 0.4%. This proves to say that OPCAB has definitely reduced the mortality of coronary surgery. And in experienced hands, it would be possible to perform OPCAB in any patient who needs CABG.
REFERENCES
- Buffolo, E., Andrade, J.C.S., Succi, J.E., et al. (1985) Direct myocardial revascularization without cardiopulmonary bypass. The Thoracic and Cardiovascular Surgeon, 33, 26-29. doi:10.1055/s-2007-1014076
- Benetti, F.J. (1985) Direct coronary surgery with saphenous vein bypass without either cardiopulmonary bypass or cardiac arrest. The Journal of Cardiovascular Surgery, 26, 217-222.
- Benetti, F.J. (1995) Video assisted coronary bypass surgery. The Journal of Cardiovascular Surgery, 10, 620- 625.
- Calafiore, A.M., Gianmarco, G.D., Teodori, G., et al. (1996) Left anterior descending coronary artery grafting via left anterior small thoracotomy without cardiopulmonary bypass. The Annals of Thoracic Surgery, 61, 1658- 1665. doi:10.1016/0003-4975(96)00187-7
- Calafiore, A.M., Vitolla, G., Mazzei, V., et al. (1998) The Last operation technique and results before and after stabilization era. The Annals of Thoracic Surgery, 66, 998- 1001. doi:10.1016/S0003-4975(98)00708-5
- Borst, C., Jansen, E.W., Tulleken, C.A., et al. (1996) Coronary artery bypass grafting without cardiopulmonary bypass and without interruption of native coronary flow using a novel anastomosis site restraining device (“Octopus”). Journal of the American College of Cardiology, 27, 1356-1364. doi:10.1016/0735-1097(96)00039-3
- Benetti, F.J., Naselli, C., Wood, M. and Geffner, L. (1991) Direct myocardial revascularization without extracorporeal circulation. Experience in 700 patients. Chest, 100, 312-316. doi:10.1378/chest.100.2.312
- Kim, K.B., Lim, C., Lee, C., et al. (2001) Off-pump coronary artery bypass may decrease the patency of saphenous vein grafts. The Annals of Thoracic Surgery, 72, 1033-1037. doi:10.1016/S0003-4975(01)02946-0
- Kim, K.B., Lim, C., Ahn, H. and Yang, J.K. (2001) Intraaortic ballon pump therapy facilitates posterior vessel off-pump coronary artery bypass grafting in high-risk patients. The Annals of Thoracic Surgery, 71, 1964-1968. doi:10.1016/S0003-4975(01)02638-8
- Puskas, J.D., Thourani, V.H., Marshall, J.J., et al. (2001) Clinical outcomes, angiographic patency and resource utilization in 200 consecutive off-pump coronary bypass patients. The Annals of Thoracic Surgery, 71, 1477-1484. doi:10.1016/S0003-4975(01)02473-0
- Shroyer, A.L., Grover, F.L., Hattler, B., Collins, J.F., McDonald, G.O., Kozora, E., Lucke, J.C., Baltz, J.H., Novitzky, D. and Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group. (2009) On-pump versus off-pump coronary-artery bypass surgery. The New England Journal of Medicine, 361, 1827-1837.
- Buckberg, G.D. and Allen, B.S. (1996) Myocardilal protection management during adult cardiac operations. In: Baue, A.E., Geha, A.S., Hammond, G.L., Laks, H. and Naunheim, K.S., Eds., Glenn’s thoracic and cardiovascular surgery, Apple & Lange, Stamford, 1653-1687.
- Mehlhorn, U., Allen, S.J., Adams, D.L., Davies, K.L., Gogola, G.R., De Vivie, R. and Laine, G.A. (1995) Normothermic continuous antegrade blood cardioplegia does not prevent myocardial edema and cardiac dysfunction. Circulation, 92, 1940-1946. doi:10.1161/01.CIR.92.7.1940
- Atkins, C.W., Boucher, C.A. and Pohot, G.M. (1984) Preservation of interventricular septal function in patient having coronary artery bypass grafts without cardiopulmonary bypass. American Heart Journal, 107, 304-309. doi:10.1016/0002-8703(84)90379-X
- Ascione, R., Lloyd, C.T., Gomes, W.J., Caputo, M., Bryan, A.J. and Angelini, G.D. (1999) Beating versus arrested heart revascularization: Evaluation of myocardial function in a prospective randomized study. European Journal Cardio-Thoracic Surgery, 15, 685-690. doi:10.1016/S1010-7940(99)00072-X
- Penttilä, H.J., Lepojärvi, M.V.K., Kiviluoma, K.T., Kaukoranta, P.K., Hassinen, I.E. and Peuhkurinen, K.J. (2001) Myocardial preservation during coronary surgery with and without cardiopulmonary bypass. The Annals of Thoracic Surgery, 71, 565-571. doi:10.1016/S0003-4975(00)02002-6
- Tashiro T., Todo K., Haruta Y., Yasunaga H., Tachikawa Y. (1996) Coronary artery bypass grafting without cardiopulmonary bypass for high-risk patients. The Journal of Cardiovascular Surgery, 4, 207-211. doi:10.1016/0967-2109(96)82317-9
- Czerny, M., Baumer, H., Kilo, J., Zuckermann, A., Grubhofer, G., Chevtchik, O., Wolner, E. and Grimm, M. (2001) Complete revascularization in coronary artery bypass grafting with and without cardiopulmonary bypass. The Annals of Thoracic Surgery, 71, 165-169. doi:10.1016/S0003-4975(00)02230-X
- Bennetts, J.S., Baker, R., Ross, I.K. and Knight, J.L. (2002) Assessment of myocardial injury by troponin T in off-pump coronary artery grafting and conventional coronary artery graft surgery. ANZ Journal of Surgery, 72, 105-109. doi:10.1046/j.1445-2197.2002.02317.x
- Masuda, M., Morita, S., Tomita, H., Kurisu, K., Nishida, T., Tominaga, R. and Yasui, H. (2002) Off-pump attenuates myocardial enzyme leakage but not postoperative brain natriuretic peptide secretion. Annals of Thoracic and Cardiovascular Surgery, 8, 139-144.
- Matata, B.M., Sosnowski, A.W. and Galiñanes, M. (2000) Off-pump bypass graft operation significantly reduces oxidative stress and inflammation. The Annals of Thoracic Surgery, 69, 785-791. doi:10.1016/S0003-4975(99)01420-4
- Guyton, R.A., Thourani, V.H., Puskas, J.D., Shanewise, J.S., Steele, M.A., Palmer-Steele, C.L. and Vinten-Johansen, J. (2000) Perfusion-assisted direct coronary artery bypass: Selective graft perfusion in off-pump cases. The Annals of Thoracic Surgery, 69, 171-175. doi:10.1016/S0003-4975(99)01386-7
- Muraki, S., Morris, C.D., Budde, J.M., Velez, D.A., Zhao, Z., Guyton, R. and Vinten-Johanson, J. (2001) Experimental off-pump coronary artery revascularization with adenosineenhanced reperfusion. The Journal of Thoracic and Cardiovascular Surgery, 121, 570-579. doi:10.1067/mtc.2001.112342
- Vassiliades, T.A., Nielsen, J.L. and Lonquist, J.L. (2002) Coronary perfusion methods during off-pump coronary bypass: Results of a randomized clinical trial. The Annals of Thoracic Surgery, 74, S1383-S1389. doi:10.1016/S0003-4975(02)03912-7
- Yeatman, M., Caputo, M., Narayan, P., Ghosh, A.K., Ascione, R., Ryder, I. and Angelini, G.D. (2002) Intracoronary shunts reduce transient intraoperative myocardial dysfunction during off-pump coronary operations. The Annals of Thoracic Surgery, 73, 1411-1417. doi:10.1016/S0003-4975(02)03407-0
- Mathison, M., Buffolo, E., Jatene, A.D., Jatene, F., Reichenspurner, H., Matheny, R.G., Shennib, H., Akin, J.J. and Mack, M.J. (2000) Right heart circulatory support facilitates coronary artery bypass without cardiopulmonary bypass. The Annals of Thoracic Surgery, 70, 1083-1085. doi:10.1016/S0003-4975(00)01827-0
- Mueller, X.M., Chassot, G., Zhou, J., Eisa, K.M., Chappuis, C., Tevaearai, H.T. and Von Seggesser, L.K. (2002) Hemodynamics optimization during off-pump coronary artery bypass: The “no compression” technique. European Journal Cardio-Thoracic Surgery, 22, 249-254. doi:10.1016/S1010-7940(02)00270-1
- Sepic, J., Wee, J.O., Soltesz, E.G., Hsin, M.K., Cohn, L.H., Laurence, R.G. and Aklog, L. (2002) Cardiac positioning using an apical suction device maintains beating heart hemodynamics. Heart Surgery Forum, 5, 279-284.
- Gundry, S.R., Romano, M.A., Shattuck, O.H., Razzouk, A.J. and Bailey, L.L. (1998) Seven-year follow-up of coronary artery bypasses performed with and without cardiopulmonary bypass. The Journal of Thoracic and Cardiovascular Surgery, 115, 1273-1278. doi:10.1016/S0022-5223(98)70209-0
- Arom, K.V., Flavin, T.F., Emery, R.W., Kshettry, V.R., Janey, P.A. and Petersen, R.J. (2000) Safety and efficacy of off-pump coronary artery bypass grafting. The Annals of Thoracic Surgery, 69, 704-710. doi:10.1016/S0003-4975(99)01510-6
- Calafiore, A.M., Di Giammarco, G., Teodori, G., Mazzei, V. and Vitolla, G. (1998) Recent advances in multivessel coronary grafting without cardiopulmonary bypass. Heart Surgery Forum, 1, 20-25.
- Cartier, R., Brann, S., Dagenais, F., Martineau, R. and Couturier, A. (2000) Systematic off-pump coronary artery revascularization in multivessel disease: Experience of three hundred cases. The Journal of Thoracic and Cardiovascular Surgery, 119, 221-229. doi:10.1016/S0022-5223(00)70176-0
- Puskas, J.D., Williams, W.H., Duke, P.G., Staples, J.R., Glas, K.E., Marshall, J.J., Leimbach, M., Huber, P., Garas, S., Sammons, B.H., McCall, S.A., Petersen, R.J., Bailey, D.E., Chu, H., Mahoney, E.M., Weintraub, W.S. and Guyton, R.A. (2003) Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: A prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. The Journal of Thoracic and Cardiovascular Surgery, 125, 797-808. doi:10.1067/mtc.2003.324
- Prabhu, A.D., Thazhkuni, I.E., Rajendran, S., Thamaran, R.A., Vellachamy, K.A. and Vettath, M.P. (2008) Mammary patch reconstruction of left anterior descending coronary artery. Asian Cardiovascular and Thoracic Annals, 16, 313-317. doi:10.1177/021849230801600412
- Prabhu, A.D., Karim, R.A., Rajendran, S., Thazhkuni, I.E., Thamaran, R.A., Vellachami, K.A. and Vettath, M.P. (2008) Vettath’s technique of long mammary patch reconstruction of a diffusely diseased left anterior descending coronaryartery without endarterectomy on the beating heart. The Heart Surgery Forum, 11, 64-67. doi:10.1532/HSF98.20071155
- Tashiro, T., Todo, K., Haruta, Y., Yasunaga, H. and Tachikawa, Y. (1996) Coronary artery bypass grafting without cardiopulmonary bypass for high-risk patients. Cardiovascular Surgery, 4, 207-211. doi:10.1016/0967-2109(96)82317-9
- Akiyama, K., Ogasawara, K., Inoue, T., Shindou, S., Okumura, H., Negishi, N. and Sezai, Y. (1999) Myocardial revascularization without cardiopulmonary bypass in patients with operative risk factors. Annals of Thoracic and Cardiovascular Surgery, 5, 31-35.
- Yokoyama, T., Baumgartner, F.J., Gheissari, A., Capouya, E.R., Panagiotides, G.P. and Declusin, R.J. (2000) Offpump versus on-pump coronary bypass in high-risk subgroups. The Annals of Thoracic Surgery, 70, 1546-1550. doi:10.1016/S0003-4975(00)01922-6
- Prifti, E., Bonacchi, M., Giunti, G., Frati, G., Proietti, P., Leacche, M., Salica, A., Sani, G. and Brancaccio, G. (2000) Does on-pump/beating-heart coronary artery bypass grafting offer better outcome in end-stage coronary artery disease patients? Journal of Cardiac Surgery, 15, 403-410. doi: 10.1111/j.1540-8191.2000.tb01300.x
- Christenson, J.T., Simonet, F., Badel, P. and Schmuziger, M. (1997) Evaluation of preoperative intra-aortic balloon pump support in high risk coronary patients. European Journal Cardio-Thoracic Surgery, 11, 1097-1103. doi: 10.1016/S1010-7940(97)00087-0
- D’Ancona, G., Karamanoukian, H., Ricci, M., Kawaguchi, A., Bergsland, J. and Salerno, T. (2001) Myocardial revascularization on the beating heart after recent onset of acute myocardial infarction. Heart Surgery Forum, 4, 74- 79.
- Locker, C., Shapira, I., Paz, Y., Kramer, A., Gurevitch, J., Matsa, M., Pevni, D. and Mohr, R. (2000) Emergency myocardial revascularization for acute myocardial infarction: Survival benefits of avoiding cardiopulmonary bypass. European Journal Cardio-Thoracic Surgery, 17, 234-238. doi: 10.1016/S1010-7940(00)00354-7
- Mohr, R., Moshkovitch, Y., Shapira, I., Amir, G., Hod, H. and Gurevitch, J. (1999) Coronary artery bypass without cardiopulmonary bypass for patients with acute myocardial infarction. The Journal of Thoracic and Cardiovascular Surgery, 118, 50-56. doi: 10.1016/S0022-5223(99)70140-6
- Nakayama, Y., Sakata, R., Ura, M. and Itoh, T. (2003) Long-term results of coronary artery bypass grafting in patients with renal insufficiency. The Annals of Thoracic Surgery, 75, 496-500. doi: 10.1016/S0003-4975(02)04380-1
- Ascione, R., Lloyd, C.T., Underwood, M.J., Gomes, W.J. and Angelini, G.D. (1999) On-pump versus off-pump coronary revascularization: Evaluation of renal function. The Annals of Thoracic Surgery, 68, 493-498. doi: 10.1016/S0003-4975(99)00566-4
- Ascione, R., Nason, G., Al-Ruzzeh, S., Ko, C., Ciulli, F. and Angelini, G.D. (2001) Coronary revascularization with or without cardiopulmonary bypass in patients with preoperative nondialysis-dependent renal insufficiency. The Annals of Thoracic Surgery, 72, 2020-2025. doi: 10.1016/S0003-4975(01)03250-7
- Güler, M., Kirali, K., Toker, M.E., Bozbuga, N., Ömeroglu, S.N., Akinci, E. and Yakut, C. (2001) Different CABG methods in patients with chronic obstructive pulmonary disease. The Annals of Thoracic Surgery, 71, 152-157. doi: 10.1016/S0003-4975(00)02250-5
- Roosens, C., Heerman, J., De Somer, F., Caes, F., Van Belleghem, Y. and Poelaert, J.I. (2002) Effects of offpump coronary surgery on the mechanics of the respiratory system, lung, and chest wall: Comparison with extracorporeal circulation. Critical Care Medicine, 30, 2430-2437. doi: 10.1097/00003246-200211000-00005
- Montague, N.T., Kouchoukos, N.T., Wilson, T.A., Bennett, A.L., Knott, H.W., Lochridge, S.K., Erath, H.G. and Clayton, O.W. (1985) Morbidity and mortality of coronary bypass grafting in patients 70 years of age and older. The Annals of Thoracic Surgery, 39, 552-557. doi: 10.1016/S0003-4975(10)61997-2
- Mullany, C.J., Darling, G.E., Pluth, J.R., Orszulak, T.A., Schaff, H.V., Ilstrup, D.M. and Gersh, B.J. (1990) Early and late results after isolated coronary artery bypass surgery in 159 patients aged 80 years and older. Circulation, 82, IV229-IV236.
- Boyd, W.D., Desai, N.D., Del Rizzo, D.F., Novick, R.J., McKenzie, F.N. and Menkis, A.H. (1999) Off-pump surgery decreases postoperative complications and resource utilization in the elderly. The Annals of Thoracic Surgery, 68, 1490-1493. doi: 10.1016/S0003-4975(99)00951-0
- Stamou, S.C., Dangas, G., Dullum, M.K., Pfister, A.J., Boyce, S.W., Bafi, A.S., Garcia, J.M. and Corso, P.J. (2000) Beating heart surgery in octogenarians: Perioperative outcome and comparison with younger age groups. The Annals of Thoracic Surgery, 69, 1140-1145. doi: 10.1016/S0003-4975(99)01430-7
- Al-Ruzzeh, S., George, S., Yacoub, M. and Amrani, M. (2001) The clinical outcome of off-pump coronary artery bypass surgery in the elderly patients. European Journal Cardio-Thoracic Surgery, 20, 1152-1156. doi: 10.1016/S1010-7940(01)00978-2
- Hoff, S.J., Ball, S.K., Coltharp, W.H., Glassford Jr., D.M., Lea IV, J.W. and Petracek, M.R. (2002) Coronary artery bypass in patients 80 years and over: Is off-pump the operation of choice? The Annals of Thoracic Surgery, 74, S1340-S1343. doi: 10.1016/S0003-4975(02)03913-9
- Vettath, M.P., Kannan, A.V., Sheen Peecheeyen, C.S., Baburajan, A.K., Vahab, A. and Sujith, M.P. (2003) Vettath’s anastamotic obturator: A simple proximal anastamotic device. The Heart Surgery Forum, 6, 366-368.
- Vettath, M.P. (2004) Vettath’s anastamotic obturator— our experience of 269 proximal anastamosis. Heart, Lung and Circulation, 13, 288-290. doi: 10.1016/j.hlc.2004.02.019
- Takanashi, S., Fukui, T., Hosoda, Y. and Shimizu, Y. (2003) Off-pump long onlay bypass grafting using left internal mammary artery for diffusely diseased coronary artery. The Annals of Thoracic Surgery, 76, 635-637. doi: 10.1016/S0003-4975(02)05024-5
- Christenson, J., Simonet, F., Badel, P. and Schmuziger, M. (1997) The effect of preoperative intra-aortic balloon pump support in patients with coronary artery disease, poor left ventricular function (LVEF < 40%) and hypertensive LV hypertrophy. The Journal of Thoracic and Cardiovascular Surgery, 45, 60-64. doi: 10.1055/s-2007-1013688
- Christenson, J., Simonet, F., Badel, P. and Schmuziger, M. (1999) Optimal timing of preoperative intraaortic balloon pump support in high-risk coronary patients. The Annals of Thoracic Surgery, 68, 934-939. doi:10.1016/S0003-4975(99)00687-6
NOTES
*Corresponding author.

