Materials Sciences and Applications
Vol. 4 No. 4 (2013) , Article ID: 30590 , 4 pages DOI:10.4236/msa.2013.44030
Synthesis, Characterization and Charge-Discharge Properties of Layer-Structure Lithium Zinc Borate, LiZnBO3
![]()
Department of Applied Chemistry, College of Bioscience and Chemistry, Kanazawa Institute of Technology Ishikawa, Ishikawa, Japan.
Email: tsuyu@neptune.kanazawa-it.ac.jp
Copyright © 2013 Isao Tsuyumoto, Akihiro Kihara. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 15th, 2013; revised February 17th, 2013; accepted March 20th, 2013
Keywords: Oxide; Borate; Lithium; Zinc; X-Ray Diffraction; Battery
ABSTRACT
Layer-Structure lithium zinc borate, LiZnBO3, is prepared by a solid state reaction of LiOH∙H2O, ZnO, and H3BO3 at 1000˚C for 10 h. Highly preferred orientation and a layer-structure are observed in the powder XRD patterns and the SEM images, respectively. The Rietveld analysis indicates a monoclinic unit cell with space group C2/c, and the lattice parameters are refined as a = 8.827 Å, b = 5.078 Å, c = 6.171 Å, and β = 118.86˚. LiZnBO3 shows the capacity of 17 mAh/g between 1.3 V and 4.3 V (vs. Li/Li+) larger than ZnO.
1. Introduction
Lithium metal phosphates (LiMPO4), lithium metal silicates (Li2MSiO4) and lithium metal borates (LiMBO3) have attracted considerable interest as cathode materials of lithium ion battery [1]. LiFePO4 with olivine structure showed the reversible extraction and insertion of lithium at 3.5 V (vs. Li) [2], and this material has been already put to practical use by some lithium ion battery manufacturers. Analogs of LiFePO4 have been explored extensively by many researchers, and the charge-discharge properties have been reported for lithium metal phosphates such as LiMnPO4, LiCoPO4 [3-5], lithium metal silicates such as Li2FeSiO4 [6-9], and lithium metal borates such as LiMnBO3, LiFeBO3 [10-15]. Polyanions with strong covalent bonds such as ,
, 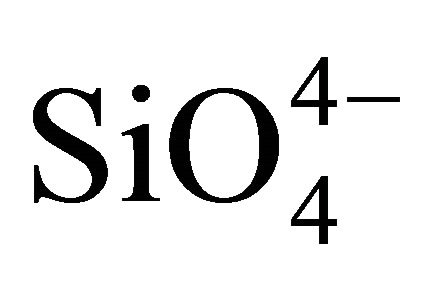 ,
,  raise transition metal redox energies through inductive effect and stabilize the structure, thereby providing high performance and chemical safety.
raise transition metal redox energies through inductive effect and stabilize the structure, thereby providing high performance and chemical safety.
LiZnBO3 was first reported by Lehman and Shadow, and its preparation and characterization have been reported by a number of researchers [16-20]. In the ternary system of Li2O-ZnO-B2O3, two polymorphs of LiZnBO3 have been reported: one prepared by solid state reaction (α-LiZnBO3) and the other from hydrothermal synthesis (β-LiZnBO3). Zinc-containing borates have been investigated aiming at the application to nonlinear optics and ferroelectrics, and the earlier reports describe the preparation of single crystals and structure analysis with a view to non-centrosymmetry. The crystal structure of α- LiZnBO3 is composed of ZnO4 tetrahedra and BO3 triangles by sharing O vertices and affords three-dimensional open channels that are occupied by lithium ions [20]. In spite of the attractive framework structure of LiZnBO3, there have been no reports on the lithium deinsertion/insertion properties of LiZnBO3. In this study, we successfully synthesized a layer-structure lithium zinc borate, α-LiZnBO3, by a conventional solid state reaction, and investigated its crystal structure, morphology, and lithium deinsertion/insertion properties.
2. Experimental
2.1. Synthesis
α-LiZnBO3 was synthesized from a stoichiometric mixture of lithium hydroxide, zinc oxide, and boric acid, i.e., 2.10 g LiOH∙H2O, 4.07 g ZnO, and 3.09 g H3BO3. The mixture was placed in an alumina boat and heated in air at 1000˚C for 10 h to yield a white powder product. In earlier reports, polycrystalline LiZnBO3 has been obtained by heating the stoichiometric mixture of ZnO and LiBO2 at 1000˚C for 12 h. After preliminary heating at 620˚C for 1 h [19] or by heating the stoichiometric mixture of Li2CO3, ZnO, and H3BO3 for 750˚C for 24 h [18], whereas single plate-like crystal of α-LiZnBO3 has been obtained in a lithium borate matrix by heating the mixture of Li2CO3, ZnO, and H3BO3 with excess amount of Li2B4O7 at 830˚C [20]. In this study, LiOH∙H2O was used as the starting material instead of Li2CO3, and the heating temperature was set at 1000˚C because the thermogravimetry and differential thermal analysis (DTA/TG, DTG- 50, Shimadzu, Tokyo, Japan) of the starting mixture in air showed a broad exothermic peak above around 750˚C.
2.2. Characterization
The powder X-ray diffraction (XRD) patterns were measured with a diffractometer (XD-D1, Shimadzu, Tokyo, Japan) using graphite-monochromatized Cu Kα radiation at 30 kV and 20 mA. The crystalline parameters were refined by the Rietveld method using the RIETAN-2000 program [21]. Impurity peaks were excluded in the refinement. The morphology of α-LiZnBO3 was observed by a scanning electron microscope (SEM, JSM-5610, JEOL, Tokyo, Japan). Each sample was ground with acetylene black and polytetrafluoroethylene (PTFE) binder into paste at a weight ratio of 84:4:12, and the paste mixture was pressed onto a nickel mesh for the lithium deinsertion/insertion measurements. The Ag/Ag+ electrode for non-aqueous solvent (RE-7, ALS Corporation Ltd., Tokyo, Japan) was used as a reference electrode, and natural graphite as a counter electrode. A 1 M LiClO4 EC/DEC solution (EC:DEC = 1:1 in volume) was used as an electrolyte. The lithium deinsertion/insertion measurements were carried out in the galvanostatic mode in the range between x = 0 and x = 0.5, where x is the Li content per formula unit, i.e., x in Li1−xZnBO3. The electrochemical capacity of samples (mAh/g) was evaluated using the weight of the active materials.
3. Results and Discussion
White powder products were obtained by the solid state reaction at 1000˚C for 10 h. The powder XRD pattern of the product and the structure refinement result are shown in Figure 1. The crystal structure was refined on the basis of monoclinic symmetry with space group C2/c similarly to α-LiZnBO3 in the earlier work [20], except for some impurity peaks. In the XRD measurements, the powder sample was solidified using glue to make each plane randomly oriented, because without the sample pretreatment with the glue the strongest (002) reflection was more than twenty times greater than the second strongest reflection due to the preferred orientation. This suggested that plate-like crystallites were formed by ani-

Figure 1. The observed XRD pattern (Iobs) of the LiZnBO3 and the pattern refined by the Rietveld method (Icalc), along with the calculated Bragg position and the residual error (Iobs-Icalc).
sotropic crystal growth and the samples were prone to orient along the a-b plane. Structure parameters and selected interatomic distances are summarized in Tables 1 and 2, respectively, and view of the crystal structure of LiZnBO3 is shown in Figure 2. The lattice parameters and monoclinic angle were calculated as a = 8.827 Å, b = 5.078 Å, c = 6.171 Å, and β = 118.86˚. In this structure, two ZnO4 tetrahedra are linked together by edgesharing (two O1 atoms) to form a Zn2O6 dimer, and the Zn2O6 dimer is linked to the six other Zn2O6 dimers by sharing oxygen vertices to form a three-dimensional framework. Boron atoms are located at the triangular void surrounded by three oxygen vertices to form BO3 triangles. Lithium atoms are located in the three-dimensional channels surrounded by Zn2O6 dimers and BO3 triangles. In this study the O2 atom was located at 8f site with occupancy of 0.5, while it was located at 4e site (0, y, 1/4) with occupancy of 1.0 in the earlier work [20]. The two O2 atoms with each occupancy of 0.5 were refined at extremely close two positions at the vertex of ZnO4 tetrahedron, as shown in Figure 2, indicating the two possibilities of ZnO4 tetrahedron shape. Such arbitrary property is probable in view of the occupancy of 0.5 for Zn1. In Figure 3, SEM images show secondary particles formed by aggregation of plate-like crystallites, and the crystallites are considered to be stacked perpendicular to the a-b plane. The present sample showed a lamellar structure consisting of plate-like crystallites stacked perpendicular to the a-b plane. This may be advantageous to lithium deinsertion/insertion because open channels in the crystallites are facing the electrolyte with large area. The conductivity of the powder compact of the present LiZnBO3 estimated from the diameter of the semicircle in the cole-cole plot was 2.12 × 10−9 Scm−1. It is much smaller than LiFeBO3, 1.52 × 10−4 Scm−1 [12], and as small as LiFePO4, 2.2 × 10−9 Scm−1 [23]. The low electric
Table 1. Structure parameters of LiZnBO3 obtained by heating at 1000˚C for 10 h. System: monoclinic. Space group: C2/c (No. 15), a = 8.827 Å, b = 5.078 Å, c = 6.171 Å, β = 118.86˚, z = 4.
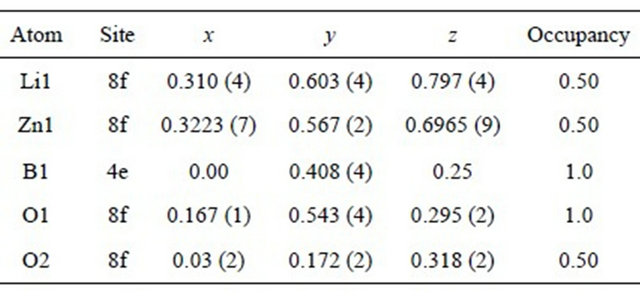
Table 2. Selected interatomic distances (Å) of LiZnBO3.

Figure 2. The crystal structure of the LiZnBO3 projected along b-axis (upper) and c-axis (lower) visualized by VESTA3 [22]. Half-filled and fully-filled circles mean the occupancies of 0.50 and 1.0, respectively.
conductivity and/or lithium diffusivity suggests the necessity to give conductivity to LiZnBO3 by treatment with carbon in order to improve the electrochemical

Figure 3. The SEM image of the layer-structure LiZnBO3. The plane facing upward is the a-b plane.
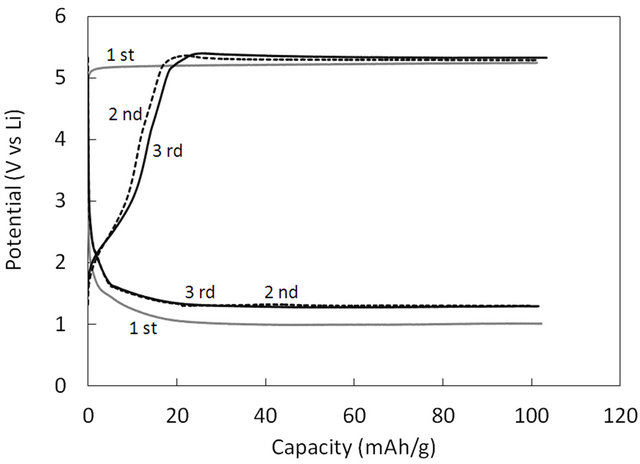
Figure 4. The charge-discharge curves of the LiZnBO3 for first three cycles at rate of 20.3 mA/g.
performance.
Figure 4 shows the typical charge and discharge curvesof LiZnBO3 at charge/discharge rate of 20.3 mA/g. The first charge curve deviated from the second and third ones, indicating that the solid electrolyte interface (SEI) was formed at the cathode/electrolyte interface during the first charge process. The capacity between 1.3 V and 4.3 V (vs. Li/Li+) was 17 mAh/g, and the charge/discharge curves showed almost the same behavior for different charge/discharge rates from 2.03 mA/g to 203 mA/g. It should be noted that our comparative experiments using ZnO as an active material did not show such charge/discharge behavior. This is probably because LiZnBO3 acted as an electric double layer capacitor (EDLC) and electric charge was accumulated at the interfacial region between the electrolyte and LiZnBO3 powder. The layer structure of LiZnBO3 was advantageous for EDLC over ZnO. The faradaic redox reaction of LiZnBO3 was not observed as opposed to the cases of LiFeBO3 and LiMnBO3 [10-15], indicating that divalent zinc was not oxidized to trivalent zinc in LiZnBO3 as well as in ZnO.
4. Conclusion
Lithium zinc borate, LiZnBO3, prepared by the solid state reaction showed a layer-structure in the SEM images. The XRD patterns well fitted to a monoclinic unit cell with space group C2/c, and the lattice parameters were refined as a = 8.827 Å, b = 5.078 Å, c = 6.171 Å, and β = 118.86˚. LiZnBO3 showed the capacity of 17 mAh/g between 1.3 V and 4.3 V (vs. Li/Li+) larger than ZnO. The capacity was due to the EDLC between the electrolyte and LiZnBO3 powder.
REFERENCES
- B. L. Ellis, K. T. Lee and L. F. Nazar, “Positive Electrode Materials for Li-Ion and Li-Batteries,” Chemistry of Materials, Vol. 22, No. 3, 2010, pp. 691-714. doi:10.1021/cm902696j
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, “Phospho-Olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries,” Journal of the Electrochemical Society, Vol. 144, No. 4, 1997, pp. 1188-1194. doi:10.1149/1.1837571
- M. Yonemura, A. Yamada, Y. Takei, N. Sonoyama and R. Kanno, “Comparative Kinetic Study of Olivine LixMPO4 (M = Fe, Mn),” Journal of the Electrochemical Society, Vol. 151, No. 9, 2004, pp. A1352-A1356. doi:10.1149/1.1773731
- D. Wang, H. Buqa, M. Crouzet, G. Deghenghi, T. Drezen, I. Exnar, N. H. Kwon, J. H. Miners, L. Poletto and M. Grätzel, “High-Performance, Nano-Structured LiMnPO4 Synthesized via a Polyol Method,” Journal of Power Sources, Vol. 189, No. 1, 2009, pp. 624-628. doi:10.1016/j.jpowsour.2008.09.077
- K. Amine, H. Yasuda and M. Yamachi, “Olivine LiCoPO4 as 4.8 V Electrode Material for Lithium Batteries,” Electrochemical and Solid-State Letters, Vol. 3, No. 4, 2000, pp. 178-179. doi:10.1149/1.1390994
- A. Nytén, A. Abouimrane, M. Armand, T. Gustafsson and J. O. Thomas, “Electrochemical Performance of Li2FeSiO4 as a New Li-Battery Cathode Material,” Electrochemical Communications, Vol. 7, No. 2, 2005, pp. 156- 160. doi:10.1016/j.elecom.2004.11.008
- A. Nytén, S. Kamali, L. Häggström, T. Gustafsson and J. O. Thomas, “The Lithium Extraction/Insertion Mechanism in Li2FeSiO4,” Journal of Materials Chemistry, Vol. 16, No. 23, 2006, pp. 2266-2272. doi:10.1039/b601184e
- M. Nadherna, R. Dominko, D. Hanzel, J. Reiter and M. Gaberscek, “Electrochemical Behavior of Li2FeSiO4 with Ionic Liquids at Elevated Temperature,” Journal of the Electrochemical Society, Vol. 156, No. 7, 2009, A619- A626. doi:10.1149/1.3133183
- C. Sirisopanaporn, C. Masquelier, P. G. Bruce, A. R. Armstrong and R. Dominko, “Dependence of Li2FeSiO4 Electrochemistry on Structure,” Journal of the American Chemical Society, Vol. 133, No. 5, 2011, pp. 1263-1265. doi:10.1021/ja109695r
- V. Legagneur, Y. An, A. Mosbah, R. Portal, A. Le Gal La Salle, A. Verbaere, D. Guyomard and Y. Piffard, “LiMBO3 (M = Mn, Fe, Co): Synthesis, Crystal Structure and Lithium Deinsertion/Insertion Properties,” Solid State Ionics, Vol. 139, No. 1-2, 2001, pp. 37-46. doi:10.1016/S0167-2738(00)00813-4
- Y. Z. Dong, Y. M. Zhao, Z. D. Shi, X. N. An, P. Fu and L. Chen, “The Structure and Electrochemical Performance of LiFeBO3 as a Novel Li-Battery Cathode Material,” Electrochimica Acta, Vol. 53 No. 5, 2008, pp. 2339-2345. doi:10.1016/j.electacta.2007.09.050
- Y. Z. Dong, Y. M. Zhao, P. Fu, H. Zhou and X. M. Hou, “Phase Relations of Li2O-FeO-B2O3 Ternary System and Electrochemical Properties of LiFeBO3 Compound,” Journal of Alloys and Compounds, Vol. 461, No. 1-2, 2008, pp. 585-590. doi:10.1016/j.jallcom.2007.07.099
- A. Yamada, N. Iwane, Y. Harada, S. Nishimura, Y. Koyama and I. Tanaka, “Lithium Iron Borates as High-Capacity Battery Electrodes,” Advanced Materials Vol. 22, No. 32, 2010, pp. 3583-3587. doi:10.1002/adma.201001039
- J. C. Kim, C. J. Moore, B. Kang, G. Hautier, A. Jain and G. Ceder, “Synthesis and Electrochemical Properties of Monoclinic LiMnBO3 as a Li Intercalation Material,” Journal of the Electrochemical Society, Vol. 158, No. 3, 2011, pp. A309-A315. doi:10.1149/1.3536532
- A. Yamada, N. Iwane, S. Nishimura, Y. Koyama and I. Tanaka, “Synthesis and Electrochemistry of Monoclinic Li(MnxFe1-x)BO3: A Combined Experimental and Computational Study,” Journal of Materials Chemistry, Vol. 21, No. 29, 2011, pp. 10690-10696. doi:10.1039/c1jm11131k
- H. A. Lehmann and H. Schadow, “Bildung und Darstellung von gemischten Monoboraten des Types MeLiBO3, (Me = Co, Zn, Mn),” Zeitschrift für Anorganische und Allgemeine Chemie, Vol. 348, No. 1-2, 1966, pp. 42-49. doi:10.1002/zaac.19663480106
- O. S. Bondareva, M. A. Simonov, Y. K. Egorov-Tismenko and N. V. Belov, “The Crystal Structures of LiZn [BO3] and LiMn[BO3],” Soviet Physics Crystallography, Vol. 23, No. 3, 1978, pp. 269-271.
- A. Belkebir, P. Tarte, A. Rulmont and B. Gilbert, “Synthesis, Structural and Vibrational Analysis of LiMBO3 Orthoborates (M = Mg, Co, Zn),” New Journal of Chemistry, Vol. 20, No. 3, 1996, pp. 311-316.
- K. S. Chang, “LiZnBO3: Crystal Structure,” Journal of the Korean Chemical Society, Vol. 45, No. 19, 2001, pp. 251- 255.
- X. Chen, C. Yang, X. Chang, H. Zang and W. Xiao, “Syntheses and Characterization of Two Alkali-Metal Zinc Borates, α-LiZnBO3 and Li0.48Na0.52ZnBO3,” Solid State Sciences, Vol. 11, No. 12, 2009, pp. 2086-2092. doi:10.1016/j.solidstatesciences.2009.08.024
- F. Izumi and T. Ikeda, “A Rietveld-Analysis Programm RIETAN-98 and Its Applications to Zeolites,” Materials Science Forum, Vol. 321-324, 2000, pp. 198-205. doi:10.4028/www.scientific.net/MSF.321-324.198
- K. Momma and F. Izumi, “VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data,” Journal of Applied Crystallography, Vol. 44, No. 6, 2011, pp. 1272-1276. doi:10.1107/S0021889811038970
- D. Wang, H. Li, S. Shi, X. Huang and L. Chen, “Improving the Rate Performance of LiFePO4 by Fe-Site Doping,” Electrochimica Acta, Vol. 50, No. 14, 2005, pp. 2955- 2958. doi:10.1016/j.electacta.2004.11.045

