Journal of Biomaterials and Nanobiotechnology
Vol.4 No.3A(2013), Article ID:33627,8 pages DOI:10.4236/jbnb.2013.43A005
Protective Effect of Phenolic-Rich Extracts from Different Parts of Opuntia joconostle Fruit against Carbon Tetrachloride-Induced Oxidative Stress in Mice*
![]()
1Departamento de Bioingenieria y Biotecnología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, México City, México; 2Departamento de Morfología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, México City, México; 3Departamento de Farmacia, Facultad de Química, Universidad Autónoma del Estado de México, Toluca, México.
Email: ortizalicia@hotmail.com
Copyright © 2013 Obed Osorio-Esquivel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 25th, 2013; revised May 28th, 2013; accepted June 8th, 2013
Keywords: Opuntia joconostle; Oxidative Stress; Carbon Tetrachloride; Phenolic Compounds
ABSTRACT
Opuntia joconostle fruit is a rich source of biocompounds such as polyphenols including gallic, vanilic, 4-hidroxybenzoic, cafeic, and syringic acids, catechin, epicatechin, rutin, and vanillin, besides betalains. The objective of this research was to evaluate the effect of supplementation polyphenols-rich extracts from different parts of Opuntia joconostle against carbon tetrachloride-induced oxidative stress in a mouse model. The animals were treated orally with polyphenols-rich extracts at 50, 100 and 200 mg/kg BW for 30 consecutive days. On day 30th the mice received carbon tetrachloride (CCl4) as hepatoxic agent. Biochemical evaluations were carried out 24 h after induction of the oxidative stress. Data showed that methanolic extracts from different parts of Opuntia joconostle exerting protective effect against the CCl4-induced oxidative stress in mice. Histology examination revealed that the damage decreased in groups treated with polyphenols-rich extracts compared to the group that did not receive any treatment. Opuntia joconostle fruit contains many phenolic compounds, flavonoids and betalains. The protective effect of extracts may be related to the phenolic composition and also by a counteraction with other compounds, such as betalains and flavonoids that increase their antioxidant effect.
1. Introduction
The fruit of Opuntia joconostle cactus is known as xoconostle (sour-prickly pear). It has a light red-pink colored pericarp, a yellow-pink, and succulent mesocarp, and a deep red colored endocarp that contains small seeds [1]. The plant produces 60 g rounded fruits with a diameter of 4 - 5 cm [2]. The xoconostle has been used in folk medicine as a treatment for diabetes, hypertension, obesity and respiratory ailments [3]. Recently, it has been reported that consumption of O. joconostle pericarp reduced hypercholesterolemia, decreased glycemic levels and increased seric insulin levels in humans [4].
Plants rich in phenolics have gained more attention due to their bioactivity [5]. O. joconostle fruits have phenolic compounds such as gallic acid, vanilic acid, 4- hidroxybenzoic acid, cafeic acid, syringic acid, catechin, epicatechin, rutin, and vanillin. Moreover pigments such as betanidin-5-Ο-β-glucoside (betanin), isobetanidin-5- Ο-β-glucoside (isobetanin), betanidin, isobetanidin and phyllocactin. These compounds have showed high antioxidant activity with DPPH+ and TEAC assays [2,6]. Epidemiological studies have suggested that high intake of phenolic compounds decreased mortality from coronary artery disease and generally decreased the risk for stroke, as well as for lung and rectal cancers, asthma and chronic obstructive pulmonary disease [7]. An imbalance caused by excess oxidants leads to oxidative stress, resulting in damage to DNA and protein and increased risk of degenerative diseases such as cancer [7,8]. Steroids, vaccines, and antiviral drugs, which have been used to treat liver diseases, have potential adverse effects, especially when administered long-term [9]. Liver injury induced by CCl4 is the most extensively studied system for xenobioticinduced oxidative stress. Reductive dehalogenation of CCl4 by the P450 enzyme system to the highly reactive trichloromethyl radical initiates the process of lipid peroxidation which is considered to be the most important mechanism in the pathogenesis of liver damage induced by CCl4 [9,10]. Thus, the aim of this study was to evaluate the protective effect of methanolic extracts from different parts of Opuntia joconostle fruit such as pericarp, mesocarp, and endocarp against CCl4-induced oxidative stress in a mouse model.
2. Materials and Methods
2.1. Plant Material
Xoconostle fruits (Opuntia joconostle var. F.A.C.Weber) were purchased from a local market in Mexico State. Voucher specimen is on deposit at the Herbarium of Centro Medico Siglo XXI, Mexico D.F. (number 15721). Five lots of 1 kg each were used for the study. Xoconostle fruits were washed, and then the pericarp, mesocarp, and endocarp were separated manually and frozen at −25˚C ± 1˚C prior to extracts preparation, seeds were discarded.
All the chemicals and solvents used in this study were purchased from sigma (Toluca, Mexico).
2.2. Preparation of Methanolic Extracts
Fresh pericarp, mesocarp, and endocarp, were extracted as described by Osorio-Esquivel et al., (2011) with slight modifications. A 1 kg of plant material was shaken with 3 L of methanol for 30 min at room temperature using an orbital shaker at 200 rpm. The mixture was vacuum filtered through Whatman No. 1 filter paper and the supernatant was placed into an amber container. The residue was re-extracted with 80% methanol and vacuum filtered using Whatman No. 1 filter paper. Then the supernatants of each part were combined and the solvent was evaporated using a rotary evaporator at 40˚C - 55˚C to remove methanol and water. The residues were evaporated to dryness in vacuum and the resulting dry powders were stored at 4˚C. Extracts were identified as MPE: methanolic pericarp extract; MME: methanolic mesocarp extract and MEE: methanolic endocarp extract. The yield of methanolic extracts was 8.56% for pericarp, 7.72% for mesocarp, and 5.26% for endocarp.
2.3. Animals
Male 8-week-old CD-1 mice (n = 72) with mean weight of 25 ± 2 g were obtained from Universidad Autonoma del Estado de Hidalgo and randomly divided into twelve groups of six animals each. Animals were administered once daily by gavages of gastric tube for 30 days. Body weight and food intake were recorded during the experimental period. Group I served as control and received only distilled water. Group II served as negative control and received the vehicle (acidified water with 0.01% citric acid). Group III (positive control antioxidant) was given ascorbic acid (200 mg/kg based in Recommended Dietary Allowance of vitamin C for a 70 kg male) [11]. Groups IV to VI were administered with MPE at doses 50, 100 and 200 mg/kg, respectively. Groups VII to IX received MME at doses 50, 100, and 200 mg/kg, respectively, and groups X to XII were administered with MEE at doses 50, 100 and 200 mg/kg. Mice were provided with Purina rodent laboratory chow diet (Cat. No. 5001), and tap water ad libitum. Experiments in animals were approved by the Laboratory Animal Care Committee of our Institution and were conducted in compliance with the Official Mexican Standards [12] regarding technical specifications for production, care and use of laboratory animals.
On the 30th day, 12 hours after the final treatment, mice were administered intragastrically with a single dose of CCl4 (0.7 mg/kg, dissolved in olive oil). Then mice were fasted 12 hours before sacrificed. Liver was dissected and weighted, and then the left lobule of each liver was fixed in 10% formalin for at least 24 h and processed by standard procedure for paraffin embedding. The lobules were sectioned into 10 µm thick sections in a rotary microtome (Microm, HM325 model, Walldorf, Germany).
Total protein content was determinate with Bradford assay. Results were calculated using a bovine albumin standard curve [13].
2.4. Liver Biochemical Assay
2.4.1. Determination of Lipid Peroxidation
Lipid peroxidation was determined by the Buege and August method [14] with slight modifications. A 200 µL aliquot of liver homogenate was added 800 µL of 150 mM Tris-HCl buffer pH 7.4 to make up 1 mL volume. This mix was incubated at 37˚C for 30 min; then 2 mL of TCA-TBA reagent (0.375% thiobarbituric acid in 15 % trichloroacetic acid) was added and the sample was vortexed. Later, the sample was heated in boiling water bath for 45 min, allowed to cool and the precipitated removed by centrifugation at 3500 rpm for 10 min. Finally, absorbance was determinate at 535 nm using a spectrophotometer (Thermo Spectronic, Genesys 10 UV/Vis, Rochester, NY, USA). Results were calculated as nMMalondialdehyde mg protein−1 using the molar extinction coefficient (MEC) of 1.56 × 105 M−1∙cm−1.
2.4.2. Determination of Super Oxide Dismutase Activity (SOD)
The SOD activity was determined according to Misra and Fridovich [15]. A 40 µL aliquot of supernatant from liver homogenate was mixed in a 1-cm cuvette with 150 µL of carbonate buffer solution (50 mM sodium carbonate and 0.1 mM EDTA) pH 10.2 and 200 µL adrenaline (30 mM). Absorbance was monitored at 480 nm, at 30 s and 5 min. SOD activity was calculated with an adrenaline standard curve. Results were expressed as IU mg protein−1.
2.5. Statistical Analysis
Body weight and liver relative weight data are expressed as mean ± SD. Biochemical parameters were analyzed using one-way analysis of variance (ANOVA) significant at P ≤ 0.001. Dunnett’s test was used to compare the mean of the groups.
3. Results
3.1. Effect of Methanolic Extracts on Body Weight and Liver Relative Weight in Mice Pretreated with Methanolic Extracts
We observed that no adverse effects were produced by methanolic extracts from different parts of Opuntia joconostle fruit. Pre-treated animals presented normal behavior, no diarrhea, aggression, depression, writhing or other physiological adverse effects during the study period. The amount of diet intake was similar in all groups (data not shown).
Body weight was registered every day and the results are informed every 5 days along 30 days of treatment with methanolic extracts (Table 1).
No significant differences were found during the first 15 days (P ≤ 0.001) among the pre-treated groups respect to control group. However, after 20 days of pre-treatment we observed a significant decrease on body weight (P ≤ 0.001) in IV, VII and VIII groups administered with MPE at 50 mg/kg dose, MME at 50 mg/kg and 100 mg/kg doses. At the end of the study we observed a significant (P ≤ 0.001) BW reduction in VII and VIII groups respect to control group. Notably, long period administration of methanolic extracts did no show adverse effects on BW. It has been reported that apple polyphenols inhibit the expression of genes involved in fatty acid synthesis that might be responsible for the extract suppression of body weight gain [9]. Thus, polyphenols such as protocatechuic acid and caffeic acid, the main components of Opuntia joconostle fruits may have an important effect on body weight loss.
Liver is the largest organ in vertebrate’s body, and is the organ where metabolism and xenobiotic excretion is carried out in it. Liver weight or size varies when an ailment appears. There were no significant differences (P ≤ 0.001) in liver relative weight among the groups pre-

Table 1. Effect of methanolic extracts from different parts of Opuntia joconostle on body weight and liver relative weight in mice.
treated with methanolic extracts. The color in most of the groups was normal, and no macroscopically injury was observed. It has been suggested that antioxidants could provide protective effects on carbon tetrachloride-induced damage and retard the index of hepatitis [16]. The highest relative weight was found in group II (CCl4). According to our results we can assume that administration of methanolic extracts from different parts of O. joconostle at doses used were appropriate and can be extrapolate on other in vivo studies.
3.2. Histopathology Studies
CCl4 induces infiltration of the lymphocytes and Kupffer cells, massive centrilobular necrosis, sinusoidal dilatation and congestion [17]. Histopathological observations of liver sections of the control group (Figure 1(I)) showed normal cellular architecture with distinct hepatic cells, sinusoidal spaces, and a central vein. Group II (CCl4 group) showed severe degenerative changes such as vacuolization and pinotic nucleic in the parenchyma cells in the centrilobular areas. The hepatic cells were found to be fatty degeneration, necrosis, cytoplasmic vacuolization and inflammation (Figure 1(II)). However, a reduction of necrosis and inflammation it was observed in groups pre-treated with MPE at 100 mg/kg dose (Figure 1(V)) similar to vitamin C group (Figure 1(III)). Groups treated with MPE at 50 mg/kg and 200 mg/kg dose (Figures 1(IV)-(VI)) showed a severe necrosis and inflammation of centrilobular vein. In groups pre-treated with MME at 50 and 100 mg/kg doses it was observed a severe necrosis, vasodilatation, hypertrophy, inflammation and congestion around of central vein (Figures 1(VII)-(VIII)). In group treated with MME at 200 mg/kg dose moderate necrosis was observed. Notably, we observed binucleated cells (Figure 1(IX)) which indicate cell regeneration.
In groups pre-treated with MEE it was observed micro-vesicular estatosis and binucleated cells. The steatosis is considering a reversible change per se of the cells. Binucleated cells imply the regeneration through mitosis and it represents a perfectly organized disorganization of cellular components whose function is the elimination of unwanted cell populations during embryogenesis and in different physiological processes [18]. Mice groups X and XI pre-treated with MEE had much less severe hepato-cellular necrosis (Figures 1(X)-(XI)). Endocarp from Opuntia joconostle is rich in betalains which are well recognized as potent antioxidants [19]; therefore the good response might be a counteraction of polyphenols and pigments. Phenolic compounds of foods posses a variety of anti-oxidant properties (inhibition of free radical-in-
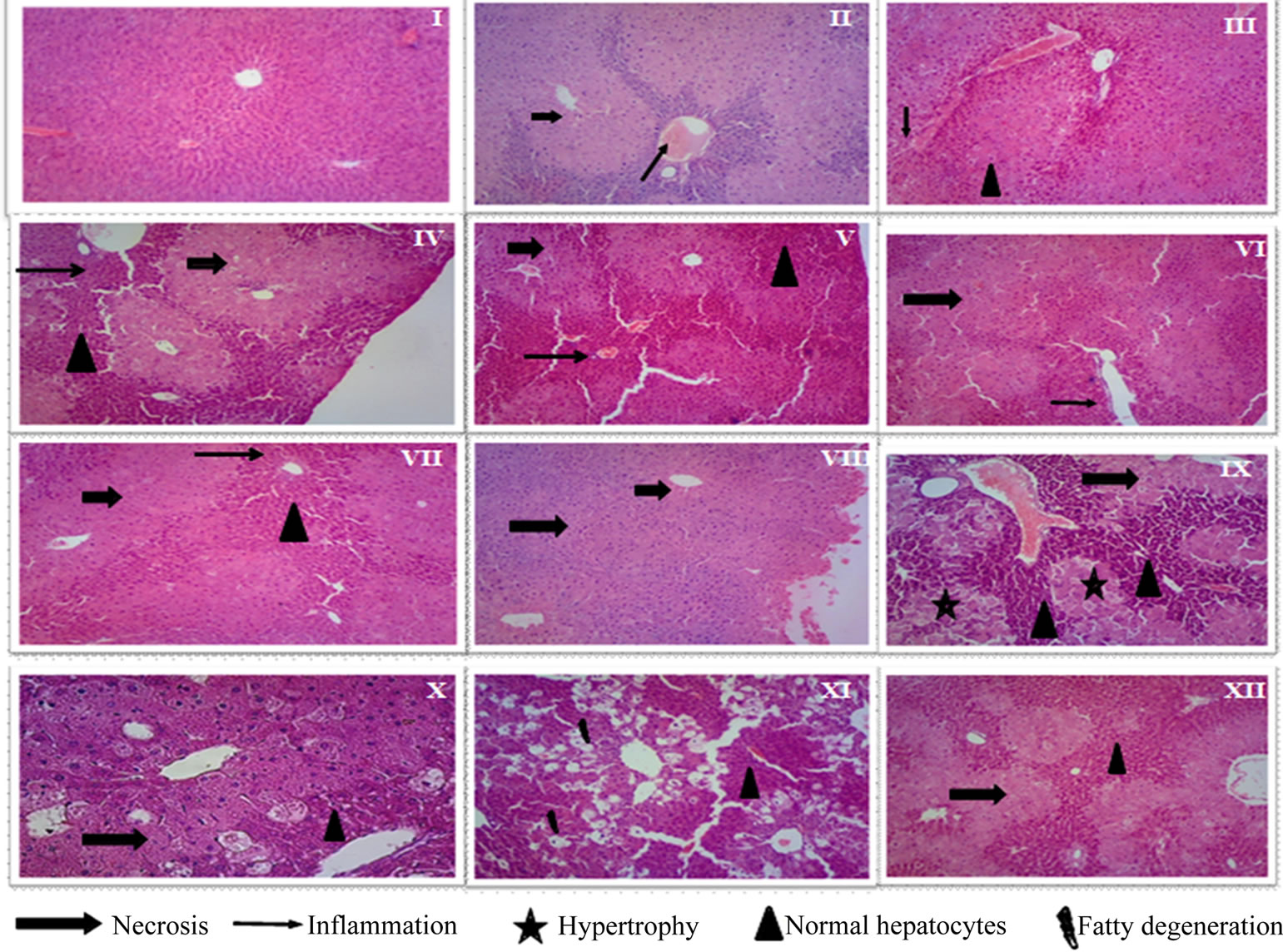
Figure 1. Effect of methanolic extracts from different parts of Opuntia joconostle on CCl4-induced liver damage of mice: (I) Control; (II) Vehicle + CCl4; (III) Ascorbic acid (200 mg/kg) + CCl4; (IV) MPE (50 mg/kg) + CCl4; (V) MPE (100 mg/kg) + CCl4; (VI) MPE (200 mg/kg) + CCl4; (VII) MME (50 mg/kg) + CCl4; (VIII) MME (100 mg/kg) + CCl4; (IX) MME (200 mg/kg) + CCl4; (X) MEE (50 mg/kg) + CCl4; (XI) MEE (100 mg/kg) + CCl4; (XII) MEE (200 mg/kg) + CCl4.
duced damage) which can be ascribed to a broad range of pharmacological activities. Antioxidant supplementation has become an attractive therapeutic strategy for reducing the risk of liver disease [9]. Finally, our results suggest that the phenolic compounds present in the O. joconostle extracts efficiently works on the liver to maintain it normally functioning, besides minimizing cell membrane damage.
3.3. Biochemical Parameters
Increased on oxygen concentration and reactive species derivates from superoxide anion (*O2−), hydroxyl radical (OH*) and H2O2 are the main causes of oxidative stress. However the body has an effective mechanism to prevent and reduce the damage caused by those free radicals. This mechanism is constituted by oxidative enzymes such as superoxide dismutase (SOD), and glutathione peroxidase (Gpx) [20]. These enzymes work in concert to detoxify superoxide anion and H2O2 in cells [21].
3.3.1. Lipid Peroxidation
Lipid peroxidation is one of the major characteristics that can be included as an oxidative damage marker [16]. Therefore, lipid peroxidation is considered to be one of the principal causes of CCl4-induced liver injury. The tricloromethyl radical reacts rapidly with oxygen from cells increasing its reactivity and forming tricloromethylperoxyl (CCl3OO*). Both radicals react with proteins and lipids, initiating the lipid peroxidation [22]. Results of pre-treatment with MPE, MME and MEE of mice are shown in Figure 2. Significant decrease (P ≤ 0.001) was
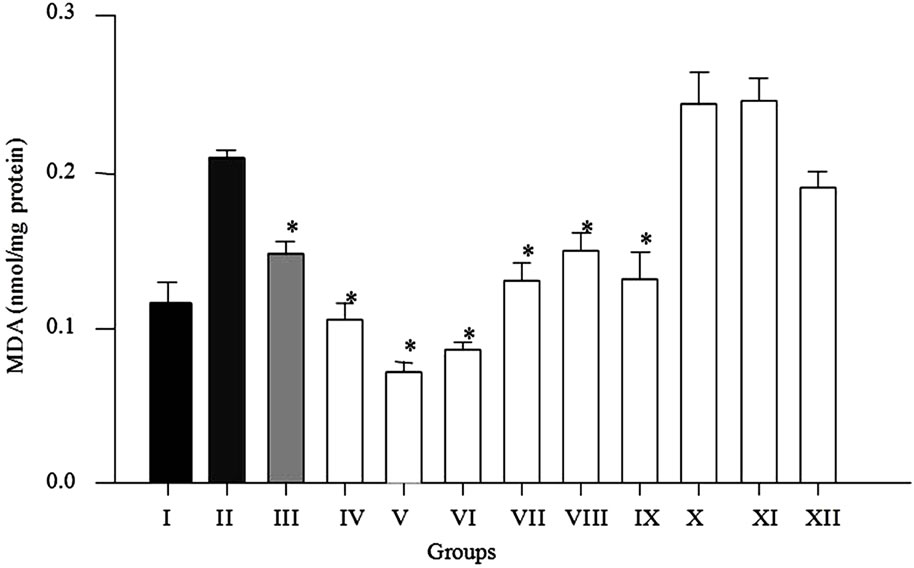
Figure 2. Pre-treatment effect of methanolic extracts on the hepatic MDA content after carbon tetrachloride-induced oxidative stress in mice. Results are expressed as mean ± standard error, n = 6, analyzed by ANOVA and Dunnett’s test. Groups-I: control; II: vehicle; III: ascorbic acid (200 mg/kg); IV: MPE (50 mg/kg) + CCl4; V: MPE (100 mg/kg) + CCl4; VI: MPE (200 mg/kg) + CCl4; VII: MME (50 mg/kg) + CCl4; VIII: MME (100 mg/kg) + CCl4; IX: MME (200 mg/kg) + CCl4; X: MEE (50 mg/kg) + CCl4; XI: MEE (100 mg/kg) + CCl4; XII: MEE (200 mg/kg) + CCl4. *P ≤ 0.001, significant difference with respect to the CCl4 group.
found in groups IV, V and VI (56.32%, 70.09% and 64.39%, respectively) respect to intoxicated group with CCl4. Group III administered with vitamin C showed 38.43% lower MDA levels respect to group II (CCl4 group). The MDA levels decrease significantly (P ≤ 0.001) in groups VII, VIII and IX pre-treated with mesocarp extract (49.33%, 37.52% and 45.21%, respectively) respect to group II. As expected extract from pericarp has better antioxidative response against CCl4 than methanolic extract from mesocarp. However an opposite behavior was observed in groups pre-treated with endocarp extract in which MDA levels increased 1.95% (X) and 2.62% (XI) respect to group II, these results may be due to high betalains content in extracts but, lower phenolic levels. It has been established that betalains should be administered in low doses about 25 - 50 µM to obtain a benefit effect, and in higher concentrations administered can be responsible of prooxidation in lipid and protein form cell wall [23]. Interestingly, we found that parts of the Opuntia joconostle richer in polyphenols (pericarp and mesocarp) give better protective effect than endocarp. It has been informed that total phenolic content in different parts of Opuntia joconostle is as follows 2.07 ± 0.01 mg/g for fresh pericarp, 1.38 ± 0.03 mg/g for fresh mesocarp, and 1.02 ± 0.03 mg/g for fresh endocarp [2]. Previously, it has been reported that the active compounds of O. ficus indica fruit juice may act by scavenging free radicals and reactive oxygen species that cause peroxidative process against the liver cells, or by inhibiting the activity of cytochrome P450 required for CCl4 metabolism [17]. Opuntia joconostle extracts decreased lipid peroxidation in liver probably through antioxidant mechanism. Lipidic oxidation due oxidative stress is responsible of injury in cell wall; consequently there is a releasing of different biomarkers of hepatic damage such as antioxidant enzymes. The range of the polyphenols extract intake in our study was 50 - 200 mg/kg BW, which is equivalent to 0.3 - 1.2 g of polyphenols for a 70 kg human adult. The total polyphenols intake could be considered to be 1 g/d as reported by Scalbert and Williamson [24]. Beneficial effect of the extracts (pericarp and mesocarp) may be due to antioxidants composition such as phenolics and flavonoids (protocatechuic acid, 4-hydoxybenzoic acid, caffeic acid, vanillic acid, syringic acid and quercetin) and pigments including betanin, isobetanin, betanidin, isobetanidin and phyllocactin [2]. Pavanato et al. (2007), reported that quercetin can act as a scavenger of CCl3 and might prevent injuries by the generated radicals causes by CCl4 [25].
3.3.2. Effect of Methanolic Extracts on SOD Activity
The antioxidant enzyme system plays an important role in the defense of cells against oxidative damage. Recently, it has been reported that antioxidant properties of flavonoides from several plant extracts posses stimulatory action and exert a stimulatory action on transcription and gene expression of certain antioxidative enzymes [21]. SOD catalyzes the dismutation of the superoxide anion to H2O2 and O2. It plays an important role in antioxidative mechanisms, this enzyme acts when there is an overproduction of free radicals due administration of CCl4 [10]. It is well-known that increased production of free radicals caused by administration of CCl4 is a major cause of the significantly reduced SOD activity [9]. Results of the effect of methanolic extracts on SOD activity are displayed in Figure 3. Treatment of mice with MPE (50, 100 and 200 mg/kg BW) showed a significant increase (P < 0.001) in SOD activity (approximately 4-fold, 5-fold and 5-fold, respectively) compared to the CCl4 group. The SOD activity decreased significantly (P < 0.05) in mice treated with MME at 50, 100 and 200 mg/kg BW (approximately 76.0%, 73.8%, and 81.9% respectively) and MEE 50, 100 and 200 mg/kg BW (approximately 76.5%, 71.6%, and 75.1% respectively) compared to the group I. However, SOD activity increased among the MME and MEE groups compared to CCl4 group. In this study, pre-treatment with methanolic extracts from MPE attenuates CCl4-induced oxidative stress in mice (Figure 3).
Administration of CCl4 to mice decreased the antioxidant capacity of the antioxidant enzymes [9]. Thus, increase of SOD activity indicates a good response to pretreatment with polyphenols-rich extracts from Opuntia
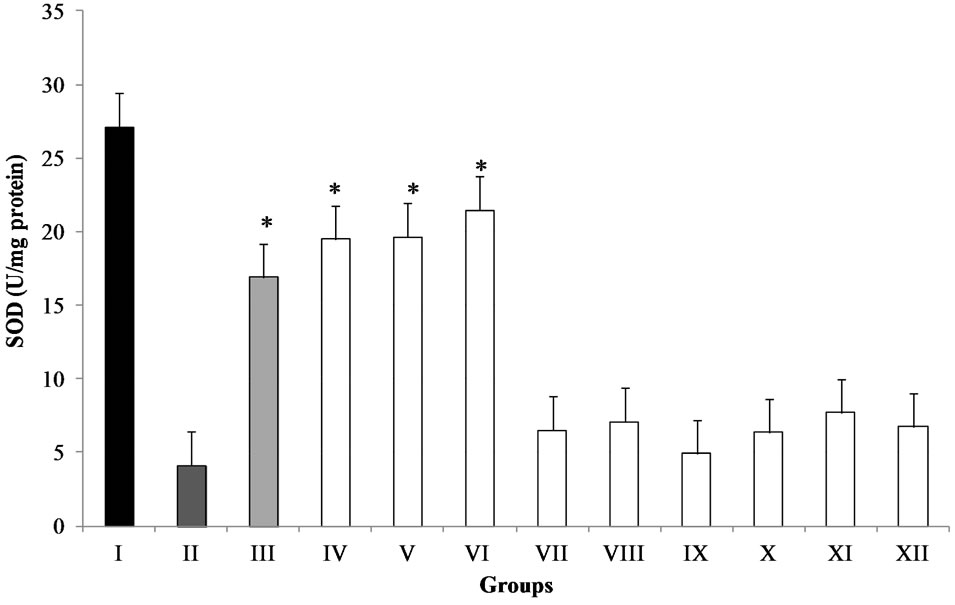
Figure 3. Effect of methanolic extracts from different parts of O. joconostle on the hepatic SOD activity after CCl4 treatment in mice. Groups-I: control; II: vehicle; III: ascorbic acid (200 mg/kg); IV: MPE (50 mg/kg) + CCl4; V: MPE (100 mg/kg) + CCl4; VI: MPE (200 mg/kg) + CCl4; VII: MME (50 mg/kg) + CCl4; VIII: MME (100 mg/kg) + CCl4; IX: MME (200 mg/kg) + CCl4; X: MEE (50 mg/kg) + CCl4; XI: MEE (100 mg/kg) + CCl4; XII: MEE (200 mg/kg) + CCl4. Results are expressed as mean ± standard error, n = 6, analyzed by ANOVA and Dunnett’s test *P ≤ 0.001 significant difference with respect to the CCl4 group.
joconostle. Previous studies have demonstrated strong relationship between total phenolic content and antioxidant activities in different varieties of crops such as eggplant, guava, onion, raspberry and Opuntia joconostle, [2,26-29].
Our study strongly suggests the antioxidant potential of the O. joconostle fruit. The results indicate that total phenolic/flavonoid content may be at least partially responsible for the antioxidant activities. Quercetin is one of the flavonoides present in O. joconostle pericarp. Several authors have reported that quercetin and other flavonoids have pharmacological properties, such as carcinostatic and antiviral activities, prevention of platelet aggregation, stabilization of immune cells, and relaxation of cardiovascular smooth muscle among other. Recently, Pavanato et al. [25] reported that quercetin significantly increases SOD and catalase activities. The results of the present study demonstrated that the synergistic action of phenolic compound from pericarp effectively protected mice against CCl4-induced oxidative stress.
4. Conclusion
In conclusion, this study indicates for the first time that phenolic-rich extracts from different parts of O. joconostle reduced damage induced by CCl4 in mice. The mechanisms of protection include the inhibition of lipid peroxidation and keeping SOD activity. However, further studies are needed to know the action mechanism and the effect of the extracts on serum biochemical parameters such as ALT and AST.
5. Acknowledgements
This research was partly funded by ConsejoNacional de Ciencia y Tecnología (CONACyT) scholarship 174690, Secretaria de Investigación y Posgrado-IPN Proyect 20110922, Comisión de Operación y Fomento de Actividades Académicas del IPN (COFAA-IPN) and Universidad Autónoma del Estado de México.
REFERENCES
- J. A. Reyes-Agüero, J. R. Aguirre and A. Valiente-Banuet, “Reproductive Biology of Opuntia: A Review,” Journal of Arid Environments, Vol. 64, No. 4, 2006, pp. 549-585. pp.10.1016/j.jaridenv.2005.06.018
- O. Osorio-Esquivel, A. Ortiz-Moreno, B. V. Álvarez, L. Dorantes-Álvarez and M. M. Giusti, “Phenolics, Betacyanins and Antioxidant Activity in Opuntiajoconostle Fruits,” Food Research International, Vol. 44, No. 7, 2011, pp. 2160-2168. pp.10.1016/j.foodres.2011.02.011
- P. Zavaleta-Becker, L. J. Olivares-Orozco, D. MontielSalero, A. Chimal-Hernandez and L. Scheinvar, “Fertilizacionorganica en Xoconostle (Opuntiajoconostle y O. matudae),” Agrociencia, Vol. 35, No. 6, 2001, pp. 609- 614.
- E. Pimienta-Barrios, L. Méndez-Moran, C. B. RamirezHernandez, E. J. García de Alba-García and M. R. Domínguez-Arias, “Effect of Xoconostle (Opuntiajoconostle Web.) Fruit Consumption on Glucose and Seric Lipids,” Agrociencia, Vol. 42, 2008, pp. 645-653.
- F. Shahidi and M. Naczk, “Phenolics in Food and Nutraceuticals,” Taylor & Francis, Boca Raton, 2004.
- S. H. Guzmán-Maldonado, A. L. Morales-Montelongo, C. Mondragón-Jacobo, G. Herrera-Hernández, F. GuevaraLara and R. Reynoso-Camacho, “Physicochemical, Nutritional, and Functional Characterization of Fruits Xoconostle (Opuntiamatudae) Pears from Central-México Region,” Journal of Food Science, Vol. 75, No. 6, 2010, pp. C485- C492. pp.10.1111/j.1750-3841.2010.01679.x
- M. Rudnicki, M. M. Silveira, T. V. Pereira, M. R. Oliveira, F. H. Reginatto, F. Dal-Pizzol and J. C. F. Moreira, “Protective Effects of Passifloraalata Extract Pretreatment on Carbon Tetrachloride Induced Oxidative Damage in Rats,” Food and Chemical Toxicology, Vol. 45, No. 4, 2007, pp. 656-661. pp.10.1016/j.fct.2006.10.022
- L.-C. Wu, H.-W. Hsu, Y.-C. Chen, C.-C. Chiu, Y.-I. Lin and J.-A. Ho, “Antioxidant and Antiproliferative Activities of Red Pitaya,” Food Chemistry, Vol. 95, No. 2, 2006, pp. 319-327. pp.10.1016/j.foodchem.2005.01.002
- J. Yang, Y. Li, F. Wang and C. Wu, “Hepatoprotective Effects of Apple Polyphenols on CCl4-Induced Acute Liver Damage in Mice,” Journal of Agricultural and Food Chemistry, Vol. 58, No. 10, 2010, pp. 6525-6531. pp.10.1021/jf903070a
- A. Srivastava and T. Shivanandappa, “Hepatoprotective Effect of the Root Extract of Decalepishamiltonii against Carbon Tetrachloride-Induced Oxidative Stress in Rats,” Food Chemistry, Vol. 118, No. 2, 2010, pp. 411-417. pp.10.1016/j.foodchem.2009.05.014
- M. Noroozi, J. W. Angerson and E. J. M. Lean, “Effects of Flavonoids and Vitamin C on Oxidative DNA Damage to Human Lymphocytes,” American Journal Clinical Nutrition, Vol. 67, 1998, pp. 1210-1218.
- D. G. Normas, “Especificaciones Técnicaspara la Produccion, Cuidado y uso de los Animales de Laboratorio,” Normas Mexicanas, 1999.
- M. M. Bradford, “A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding,” Analytical Biochemistry, Vol. 72, 1976, pp. 248-254. pp.10.1016/0003-2697(76)90527-3
- A. J. Buege and D. S. Aust, “Microsomal Lipid Peroxidation,” Methods in Enzymology, Vol. 52, 1978, pp. 302- 310. pp.10.1016/S0076-6879(78)52032-6
- P. H. Misra and I. Fridovich, “The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase,” The Journal of Biological Chemistry, Vol. 247, 1972, pp. 3170-3175.
- C.-P. Lee, P.-H. Shih, C.-L. Hsu and G.-C. Yen, “Hepatoprotection of Tea Seed Oil (Camellia oleifera Abel.) against CCl4-Induced Oxidative Damage in Rats,” Food and Chemical Toxicology, Vol. 45, No. 6, 2007, pp. 888- 895. pp.10.1016/j.fct.2006.11.007
- E. Galati, M. Mondello, E. Lauriano, M. Taviano, M. Galluzzo and N. Miceli, “Opuntiaficusindica (L.) Mill. Fruit Juice Protects Liver from Carbon Tetrachloride-Induced Injury,” Phytotherapy Research, Vol. 19, No. 4, 2005, pp. 796-800. pp.10.1002/ptr.1741
- S. R. Cotran, V. Kumar and T. Collins, “Robbins Pathologic Basis of Disease,” 6th Edition, McGraw-Hill Interamericana, 2000.
- M. Kujawska, E. Ignatowicz, M. Murias, M. Ewertowska, K. Mikolajczyk and J. Jodynis-Liebert, “Protective Effect of Red Beetroot against Carbon Tetrachloride and N-Nitrosodiethylamine-Induced Oxidative Stress in Rats,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 6, 2009, pp. 2570-2575. pp.10.1021/jf803315d
- C.-H. Huang, Y.-T. Tung, K.-C. Cheng and J.-H. Wu, “Phytocompounds from Vitiskelungensisstem Prevent Carbon Tetrachloride-Induced Acute Liver Injury in Mice,” Food Chemistry, Vol. 125, 2011, pp. 726-731. pp.10.1016/j.foodchem.2010.09.085
- S. Sreelatha, R. Padma and M. Umadevi, “Protective Effects of Coriandrumsativum Extracts on Carbon Tetrachloride-Induced Hepatotocity in Rats,” Food and Chemical Toxicology, Vol. 47, 2009, pp. 702-708. pp.10.1016/j.fct.2008.12.022
- B. Huang, X. Ban, J. He, J. Tong, J. Tian and Y. Wang, “Hepatoprotective and Antioxidant Activity of Ethanolic Extracts of Edible Lotus (Nelumbonucifera Gaertn.) Leaves,” Food Chemistry, Vol. 120, No. 3, 2010, pp. 873- 878. pp.10.1016/j.foodchem.2009.11.020
- J. Kanner, S. Harel and R. Granit, “Betalains—A New Class of Dietary Cationized Antioxidants,” Journal of Agricultural and Food Chemistry, Vol. 49, No. 11, 2001, pp. 5178-5185. pp.10.1021/jf010456f
- A. Scalbert and G. Williamson, “Dietary Intake and Bioavailability of Polyphenols,” The Journal of Nutrition, Vol. 130, No. 8, 2000, pp. 2073S-2085S.
- M. Pavanato, N. Marroni, C. Marroni and S. Llesuy, “Quercetin Prevents Oxidative Stress in Cirrhotic Rats,” Digestive Diseases and Sciences, Vol. 52, No. 10, 2007, pp. 2616-2621. pp.10.1007/s10620-007-9748-x
- P. Akanitapichat, K. Phraibung, K. Nuchklang and S. Prompitakkul, “Antioxidant and Hepatoprotective Activities of Five Eggplant Varieties,” Food and Chemical Toxicology, Vol. 48, No. 10, 2010, pp. 3017-3021. pp.10.1016/j.fct.2010.07.045
- K. Thaipong, U. Boonprakob, K. Crosby, L. CisnerosZevallos and D. H. Byrne, “Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts,” Journal of Food Composition and Analysis, Vol. 19, No. 6, 2006, pp. 669-675. pp.10.1016/j.jfca.2006.01.003
- J. Yang, K. J. Meyers, J. van der Heide and R. H. Liu, “Varietal Differences in Phenolic Content and Antioxidant and Antiproliferative Activities of Onions,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 22, 2004, pp. 6787-6793. pp.10.1021/jf0307144
- M. Liu, X. Q. Li, C. Weber, C. Y. Lee, J. Brown and R. H. Liu, “Antioxidant and Antiproliferative Activities of Raspberries,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 10, 2002, pp. 2926-2930. pp.10.1021/jf0111209
NOTES
*Conflict of interest statement: the authors declare that there are no conflicts of interest.

