Journal of Agricultural Chemistry and Environment
Vol.05 No.01(2016), Article ID:64057,7 pages
10.4236/jacen.2016.51005
Phosphate Sorption in Water by Several Cationic Polymer Flocculants
Timothy S. Goebel1, Robert J. Lascano1*, Todd A. Davis2
1Cropping Systems Research Laboratory, USDA-ARS, Lubbock, TX, USA
2Department of Chemistry, United States Air Force Academy, Colorado Springs, CO, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 24 November 2015; accepted 26 February 2016; published 29 February 2016
ABSTRACT
Although inorganic phosphate is an essential plant nutrient, elevated levels in surface waters lead to adverse effects in the environment. These effects are attributed to runoff from rain or irrigation events that may cause the sorbed phosphate to be transported from the application sites and to move into neighboring watersheds. Increased phosphate concentration in watersheds may lead to a variety of environmental problems including increased algal blooms, bacterial contamination, and in some cases eutrophication. To overcome these effects, polymer flocculants have been shown to reduce the phosphate concentration in water by removing suspended solids and thereby removing the phosphate sorbed to the solids. The purpose of this study is to determine the amount, if any, of phosphate removed by several commercial polymers. The polymers chosen include the polyacrylamides Magnifloc 494C, Magnifloc 985N and Poly (diallyldimethyl ammonium chloride) (Poly (DADMAC)). Using these polymers, it is discovered that the positive charge density of the polymers affects the amount of phosphate removed from solution with Poly (DADMAC) (having 100% positive charge density) removing 40% of the phosphate from a solution containing 10 ppm phosphate.
Keywords:
Polymer Flocculants, Phosphate, Sorption, Polyacrylamides, Poly (DADMAC), Smectite

1. Introduction
Phosphorus is an essential plant nutrient that is commonly applied as a fertilizer and is frequently found in surface water. Inorganic phosphate contamination leads to a variety of environmental problems with water resources including increased algal blooms, bacterial contaminations, and eutrophication of surface water bodies as well as rivers and streams [1] -[5] . The source of phosphate can come from runoff from agricultural fields, land application of wastewater from concentrated animal feeding operations, and municipal wastewater [2] [4] [5] . To help alleviate these adverse effects of phosphate on the environment, methods for the removal of inorganic phosphate from water are needed.
While the removal of phosphate from water is a topic of recent interest, the sorption of phosphate onto polymer flocculants has not been fully investigated [2] [6] -[11] . Polymer flocculants are routinely used to remove solid material from water [12] . They are added to municipal as well as industrial wastewater to remove suspended solids prior to further treatment to remove dissolved contaminants. These polymer flocculants interact with the surface of suspended solids, organizing the individual particles into larger structures called flocs. The flocs then settle out of solution faster as they have a larger diameter than the individual particles. Polymer flocculants can remove contaminants that are bound or incorporated into the suspended solids through the settling process. Recent work by our team has shown that polymer flocculants can be modified to increase the polymers ability to sequester phosphate from solution [7] . The results from that study show that the polymer flocculants are able to remove phosphate as well, but at a lower level than the modified polymers that are developed for that study. However, the commercially available polymers do show a trend indicating that increasing the positive charge density of the polymer results in increased phosphate removal from solution. It is the effect of positive charge density on the amount of phosphate removed from solution that is the focus of this study.
2. Materials and Methods
Surface waters have many components including both dissolved and particulate matter. For this study, smectite was chosen to represent the particulate matter present in surface waters as it is a common mineral that has been shown to have slight 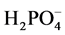 sorption [13] . Deionized water was used to control the amount of phosphate present, and NaH2PO4 (Sigma-Aldrich S8282-550G) was added to create aqueous solutions with different
sorption [13] . Deionized water was used to control the amount of phosphate present, and NaH2PO4 (Sigma-Aldrich S8282-550G) was added to create aqueous solutions with different 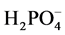 concentrations.
concentrations.
Cationic and nonionic polymers were chosen for this study. The cationic polymers evaluated were polydiallyldimethylammonium Chloride (100% positive charge density), Magnifloc 494C (10% positive charge density), and A14 (40% positive charge density). A14 was a polyacrylamide made in-house following the synthesis described in our previous work [7] . Magnifloc 985N was used as a control polymer with no positive charge (Figure 1).
Three variables were evaluated in this study. First, the amount of smectite was varied to determine the effect on the sequestration of 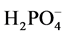 by the polymers. Second, the amount of time the mixture was stirred was varied to determine if there was a time dependent
by the polymers. Second, the amount of time the mixture was stirred was varied to determine if there was a time dependent 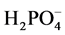 sorption maximum for the polymers. Third and finally, the amount of
sorption maximum for the polymers. Third and finally, the amount of 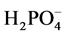 in solution was varied to determine the sorption maximum.
in solution was varied to determine the sorption maximum.
2.1. Effect of Increasing the Amount of Smectite Added
Smectite was weighed and added to a 25 mL glass Erlenmeyer flask (Kimble KIMAX Flask with ST16 Glass Stopper, Graduated, 26,600) based on the amounts shown in Table 1. 18.5 mL of deionized water was then
Figure 1. Chemical structures of polymer flocculants.
Table 1. Experimental conditions.
added to the flask, which was sealed using a glass stopper. The mixture was vortexed for 5 s and the resulting mixture was left for 24 hours to allow the smectite to swell and minimize its phosphate sorption. To this mixture 0.5 mL of 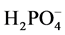 added a 10 ppm
added a 10 ppm  concentration. The mixture was vortexed for 5 s. To this mixture 1 mL of the polymer solution was added to make the final polymer concentration of 100 ppm and a final volume 20 mL. The mixture was then vortexed again for 5 s, a stir bar was added to the flask, and sealed with a glass stopper and then placed on a stir plate (JEIO TECH, Multi Channel Stirrer, Model: MS-53M) at 250 RMP for 24 hours. Two control experiments were examined. The first control was to examine the amount of
concentration. The mixture was vortexed for 5 s. To this mixture 1 mL of the polymer solution was added to make the final polymer concentration of 100 ppm and a final volume 20 mL. The mixture was then vortexed again for 5 s, a stir bar was added to the flask, and sealed with a glass stopper and then placed on a stir plate (JEIO TECH, Multi Channel Stirrer, Model: MS-53M) at 250 RMP for 24 hours. Two control experiments were examined. The first control was to examine the amount of 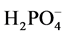 removed by the smectite. This control was prepared as above but with the addition of 1 mL of water in place of the polymer. The second control contained only
removed by the smectite. This control was prepared as above but with the addition of 1 mL of water in place of the polymer. The second control contained only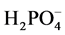 , no smectite, and 1 mL of water was added in place of the polymer. After 24 hours, the stir plate was stopped, the stir bars were removed and 8 mL of solution were placed in a centrifuge tube. The solutions were then centrifuged (Eppendorf Centrifuge 5810) at 3500 RPM for 15 min to remove any solid material that might be present. The supernatant was then filtered through a 0.2 μm syringe filter and analyzed for
, no smectite, and 1 mL of water was added in place of the polymer. After 24 hours, the stir plate was stopped, the stir bars were removed and 8 mL of solution were placed in a centrifuge tube. The solutions were then centrifuged (Eppendorf Centrifuge 5810) at 3500 RPM for 15 min to remove any solid material that might be present. The supernatant was then filtered through a 0.2 μm syringe filter and analyzed for 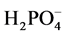 using a Dionex ICS 2100 ion chromatography system (Suppressor, AERS 500 2 mm, Guard and Analytical Column, AG18, AS18) with a 25 μm injection loop, running at a flow rate of 0.25 mL/min in gradient mode (23 mM KOH for 13 min then 30 mM KOH for 12 min) resulting in an elution time for phosphate of 23 min.
using a Dionex ICS 2100 ion chromatography system (Suppressor, AERS 500 2 mm, Guard and Analytical Column, AG18, AS18) with a 25 μm injection loop, running at a flow rate of 0.25 mL/min in gradient mode (23 mM KOH for 13 min then 30 mM KOH for 12 min) resulting in an elution time for phosphate of 23 min.
2.2. Effect of Increasing 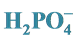 Concentration
Concentration
150 mg of smectite was weighed and added to a 25 mL glass Erlenmeyer flask (Kimble KIMAX Flask with ST16 GlassStopper, Graduated, 26,600). 18.5 mL of deionized water was then added to the flask, which was sealed using a glass stopper. The mixture was vortexed for 5 s and the resulting mixture was left for 24 hours to allow the smectite to swell and minimize its phosphate sorption. To this mixture 0.5 mL of a 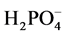 spike added to obtain the various
spike added to obtain the various 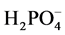 concentrations listed in Table 1. The mixture was vortexed for 5 s. To this mixture 1 mL of the polymer solution was added to make the final polymer concentration of 100 ppm and a final volume 20 mL. The mixture was then vortexed again for 5 s, a stir bar was added to the flask, and sealed with a glass stopper and then placed on a stir plate (JEIO TECH, Multi Channel Stirrer, Model: MS-53M) at 250 RMP for 24 hours. Two control experiments were examined. The first control was to examine the amount of H2PO4? removed by the smectite. This control was prepared as above but with the addition of 1 mL of water in place of the polymer. The second control contained only
concentrations listed in Table 1. The mixture was vortexed for 5 s. To this mixture 1 mL of the polymer solution was added to make the final polymer concentration of 100 ppm and a final volume 20 mL. The mixture was then vortexed again for 5 s, a stir bar was added to the flask, and sealed with a glass stopper and then placed on a stir plate (JEIO TECH, Multi Channel Stirrer, Model: MS-53M) at 250 RMP for 24 hours. Two control experiments were examined. The first control was to examine the amount of H2PO4? removed by the smectite. This control was prepared as above but with the addition of 1 mL of water in place of the polymer. The second control contained only

2.3. Effect of Mixing Time of 
150 mg of Smectite was weighed and added to a 25 mL glass Erlenmeyer flask (Kimble KIMAX Flask with ST16 GlassStopper, Graduated, 26,600). 18.5 mL of deionized water was then added to the flask, which was sealed using a glass stopper. The mixture was vortexed for 5 s and the resulting mixture was left for 24 hours to allow the smectite to swell and minimize its phosphate sorption. To this mixture 0.5 mL of 




3. Results and Discussion
3.1. Effect of Increasing the Amount of Smectite Added
The first experiment conducted was to determine the effect of increasing the amount of smectite added to the mixture. It was assumed that as more smectite was added less of the polymer would be available to interact with the H2PO4? in solution, as it would be adhered to the surface of the smectite particles. The results initially support this assumption as the polyacrylamides generally removed less 







The results from the polymers up to the 100 mg smectite addition was likely caused by saturation of the negative charge of the clay by the positive charge of the polymer. When there is a high enough concentration of the cationic polymer flocculant compared to the solid material present the charge of the solid material can become negated, thereby changing net charge of the clay-polymer complex [6] [14] [15] . This has the effect of impairing the polymers ability to form flocs and settle out of solution. The net result of this oversaturation of solid material is dispersion as the now neutral or positively charged smectite-polymer complexes repel each other. Given the smallest amount of smectite added (25 mg to 100 mg), less of the polymer was available to interact with 




3.2. Effect of Increasing H2PO4? Concentration
As the solution concentration of 










3.3. Effect of Mixing Time of 
The third and final study examined the effect of mixing time on the amount of 






Figure 2. The effect of increasing the amount of smectite added to each flask from 25 mg to 500 mg.
Figure 3. Effect of increasing the solution concentration of 

Figure 4. Effect of allowing the mixture to stir for increasing amounts of time on the amount of 
erally removed similar amounts of


4. Conclusions
The results from these studies show that it is possible to remove 











The total amount of 




Acknowledgements
This research was supported in part by the Ogallala Aquifer Program, a consortium between USDA-Agricultural Research Service, Kansas State University, Texas A&M AgriLife Research, Texas A&M AgriLife Extension Service, Texas Tech University, and West Texas A&M University.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program.
Cite this paper
Timothy S.Goebel,Robert J.Lascano,Todd A.Davis, (2016) Phosphate Sorption in Water by Several Cationic Polymer Flocculants. Journal of Agricultural Chemistry and Environment,05,45-51. doi: 10.4236/jacen.2016.51005
References
- 1. Correll, D.L. (1998) The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review. Journal of Environmental Quality, 27, 261-266. http://dx.doi.org/10.2134/jeq1998.00472425002700020004x
- 2. Kugimiya, A. and Takei, H. (2008) Selective Recovery of Phosphate from River Water Using Molecularly Imprinted Polymers. Analytical Letters, 41, 302-311. http://dx.doi.org/10.1080/00032710701792919
- 3. Livingstone, D.A. and Fleischer, M. (1963) Data of Geochemistry. US Government Printing Office.
- 4. Maguire, R.O., Sims, J.T. and Coale, F.J. (2000) Phosphorus Solubility in Biosolids-Amended Farm Soils in the Mid- Atlantic Region of the USA. Journal of Environmental Quality, 29, 1225-1233. http://dx.doi.org/10.2134/jeq2000.00472425002900040028x
- 5. Pote, D.H., Daniel, T.C., Moore, P.A., Nichols, D.J., Sharpley, A.N. and Edwards, D.R. (1996) Relating Extractable Soil Phosphorus to Phosphorus Losses in Runoff. Soil Science Society of America Journal, 60, 855-859. http://dx.doi.org/10.2136/sssaj1996.03615995006000030025x
- 6. Bolto, B. and Gregory, J. (2007) Organic Polyelectrolytes in Water Treatment. Water Research, 41, 2301-2324. http://dx.doi.org/10.1016/j.watres.2007.03.012
- 7. Goebel, T.S., McInnes, K.J., Senseman, S.A., Lascano, R.J., Marchand, L.S. and Davis, T.A. (2011) Modifying Polymer Flocculants for the Removal of Inorganic Phosphate from Water. Tetrahedron Letters, 52, 5241-5244. http://dx.doi.org/10.1016/j.tetlet.2011.07.130
- 8. Kugimiya, A. and Takei, H. (2008) Selectivity and Recovery Performance of Phosphate-Selective Molecularly Imprinted Polymer. Analytica Chimica Acta, 606, 252-256. http://dx.doi.org/10.1016/j.aca.2007.11.025
- 9. Muljadi, D., Posner, A.M. and Quirk, J.P. (1966) The Mechanism of Phosphate Adsorption by Kaolinite, Gibbsite, and Pseudoboehmite. Journal of Soil Science, 17, 212-228. http://dx.doi.org/10.1111/j.1365-2389.1966.tb01467.x
- 10. Oguz, E., Gürses, A. and Canpolat, N. (2003) Removal of Phosphate from Wastewaters. Cement and Concrete Research, 33, 1109-1112. http://dx.doi.org/10.1016/S0008-8846(03)00016-4
- 11. Patel, J. and Sudhakar, P. (2001) Phosphate Removal from Aqueous Solutions Using Mango Seed Powder. Journal of Industrial Pollution Control, 17, 213-218.
- 12. Kawamura, S. (1976) Considerations on Improving Flocculation (PDF). Journal-American Water Works Association, 68, 328-336.
- 13. Violante, A. and Pigna, M. (2002) Competitive Sorption of Arsenate and Phosphate on Different Clay Minerals and Soils. Soil Science Society of America Journal, 66, 1788-1796. http://dx.doi.org/10.2136/sssaj2002.1788
- 14. Gregory, J. (1973) Rates of Flocculation of Latex Particles by Cationic Polymers. Journal of Colloid and Interface Science, 42, 448-456. http://dx.doi.org/10.1016/0021-9797(73)90311-1
- 15. Kasper, D.R. (1971) Theoretical and Experimental Investigations of the Flocculation of Charged Particles in Aqueous Solutions by Polyelectrolytes of Opposite Charge, California Institute of Technology.
- 16. Broughton, A., Pratt, S. and Shilton, A. (2008) Enhanced Biological Phosphorus Removal for High-Strength Wastewater With a Low rbCOD:P Ratio. Bioresource Technology, 99, 1236-1241. http://dx.doi.org/10.1016/j.biortech.2007.02.013
NOTES
*Corresponding author.







