 Vol.3, No.5, 371-378 (2011) Natural Science http://dx.doi.org/10.4236/ns.2011.35050 Copyright © 2011 SciRes. OPEN ACCESS Comparative study of thermophilic and mesophilic anaerobic treatment of purified terephthalic acid (PTA) wastewater Michael Olawale Daramola1*, Elizabeth Funmilayo Aransiola1, Adeniyi Ganiyu Adeogun2 1Department of Chemical Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria; *Corresponding Author: kennydara@yahoo.com 2Department of Integrated Urban Engineering, UNESCO-IHE Institute of Water Education, Delft, The Netherlands. Received 11 February 2011; revised 5 March 2011; accepted 27 March 2011. ABSTRACT The paper provides a critical comparison be- tween mesophilic and thermophilic anaerobic treatment of PTA wastewater through diagnosis of a case study. Aspects covered are bioavail- ability, biodegradability, microbial population, thermodynamics, kinetics involved and bio- reactor design for PTA wastewater treatment. The results of the case study suggests that one- stage thermophilic anaerobic reactor coupled with coagulation-flocculation pre-treatment unit and an aerobic post treatment unit could be techno-economically viable for PTA wastewater treatment to ensure that the final effluent quality conforms to the international standard. The in- formation emanated from this study could be useful and thought provoking to the profes- sionals and academia in the area of PTA waste- water treatment and can serve as impetus to- ward the development of research lines in similar problems like the treatment of other petro- chemical wastewater such as phenol-containing wastewater, benzene/benzoic acid-containing wastewater or wastewater from other similar in- dustrial settings. Keywords: Terephthalic Acid; Wastewater Treatment; Anaerobic and Aerobic Treatment; Mesophilic and Thermophilic Conditions; Bioreactors 1. INTRODUCTION In 1997, worldwide production of purified terephthal- ic acid (PTA) was 18.22 million tons and it grew steadily to about 26.12 million tons in 2002 at an annual growth rate of 7.5% with China growth rate accounted for about 2.6 million tons. In 2005, the demand for PTA in China rose up to 12.14 million tons contributing to 42% of the total world demand of about 28.8 million tones [1,2]. The process for the production of PTA developed by the American Amoco Group [3] comprises: Wet oxidation of p-Xylene into acetic acid to pro- duce crude terephthalic acid (CTA). Hydrogenation of CTA into PTA over palladium as a catalyst [4]. During the process, about 4 - 10 kg COD·m−3 are generated with 5 - 20 COD·L−1, and 3 - 4 m3 wastewater per ton of PTA generated [5]. Significant part of PTA wastewater consists of p-toluic acid (p-Tol), benzoic acid (BA), 4-carboxybenzaldehyde (4-CBA), phthalic acid (PA) and terephthalic acid (TA) with minor concentra- tions of 4-formylbenzoic acid, methyl acetate and p- xylene [6-8]. At times, the contribution of these chemi- cals towards COD can be more than 75% in the waste- water. With increasing PET consumption, the treatment of PTA wastewater and contaminated environments is very essential to preserve natural ecosystems and protect the environment because untreated PTA waste water released into the environment is toxic to living organ- isms [9-12]. Acute toxicity, sub-acute toxicity, chronic toxicity and molecular toxicity have been reported for exposure to pure chemical PTA [11,13-16]. Additionally, phthalate, its ester and degradation in- termediates are suspected to cause cancer and renal damage, and as a result of this, the US Environmental Protection Agency has recently added this class of chemicals to the list of priority pollutants [17]. The toxic concentrations or doses of the pure chemical PTA were as high as over 1000 mg·L−1. Several methods such as, advanced oxidation processes (AOP), supercritical water oxidation, UV-assisted ozonation (UV/O3), ozone as- sisted photochemical oxidation (UV/O3/H2O2), pho- to-fenton oxidation(UV/H2O2/FeSO4), ozone-assisted pho-  M. O. Daramola et al. / Natural Science 3 (2011) 371-378 Copyright © 2011 SciRes. OPEN ACCESS 372 to-fenton oxidation (UV/O3/H2O2/FeSO4) and radiation treatment using gamma-ray have been used for the treatment of PTA wastewater [18-21]. However, cost for treatment and generation of toxic intermediates and sludge, which in turn cause secondary pollution, have been identified as major limitations of these methods. To overcome these limitations, biodegradation technology has been proposed and used. Biodegradation or biological treatment has been found to be environmental friendly and cost effective and in some cases, energy-efficient compared to chemical pro- cesses. However in biodegradation, poor degradation of the intermediate poses a problem [22-24]. Nevertheless, this problem does not pose serious hindrance to the ap- plication of biodegradation to wastewater treatment. Regarding the application of biodegradation technology for PTA wastewater treatment, activated sludge process (aerobic treatment) has been proposed and used [23]. Advantages of aerobic treatment are high purification efficiency (>90%), high process stability and rapid bio- degradability of all compounds. Meanwhile, in recent years, anaerobic treatment has been preferred to conven- tional aerobic activated sludge treatment process due to the reasons presented in Table 1. In light of this, the objective of this paper is to present a critical comparison between aerobic and anaerobic degradation of PTA wastewater with a view to furnishing the readers with useful information on PTA wastewater treatment and provoking their thought toward the appli- cation of the process to wastewater treatment from simi- lar industrial settings. Furthermore, the comparison is presented through the diagnosis of a scenario which concerns a PTA wastewater containing terephthalate, benzoate and acetate. The concentrations of the tere- phthalate, benzoate and acetate in the waste stream are 3, Table 1. Comparison between aerobic activated sludge and anaerobic degradation of wastewater. Aerobic activated sludge Anaerobic degradation High energy intensive Low energy intensive Large volume of sludge Small volume of sludge Requires highly skilled operation and process control Requires moderate skilled operation and process control Additional nutrients is required No additional nutrients required Costly technical specifications No costly technical specifications Poor solid settleability Good solid settleabiblity Requires high hydraulic retention time (HRT) Low HRT Low elimination rate High elimination rate 2 and 2 g·L−1, respectively. The wastewater leaves the plant at a temperature of 54˚C - 60˚C and a pH of 5. Thus this paper compares mesophilic and thermophilic anaerobic degradation of the wastewater and suggests an economically viable option for the treatment of the wastewater. In the course of the diagnosis, issues like bioavailability, biodegradability, microbial population, kinetics and thermodynamics of anaerobic degradation of PTA wastewater were considered and discussed. 2. BIOAVAILABILITY, BIODEGRADABILITY AND TOXICITY OF TA Terephthalic acid is a benzene ring structure with two carboxylic acid groups. These two acid groups are re- sponsible for the high solubility of TA in water. It is known that TA has a high solubility of about 140 g·L−1 at pH > 5.5 [25]. Therefore, bioavailability of TA for the degrading bacteria is promising. However, the pH should be > 5.5 or else precipitation of TA might occur. When the pH < 5.5, TA precipitation occurs, and the TA will be unavailable anymore for degradation. Kleerebezem et al. [26] have proposed a two-step biodegradation pathway for PTA wastewater. In this pathway, the first step in- volves the decarboxylation of TA to form benzoate and the second step involves the transformation of benzoate to carboxycyclohexane. The two carboxylic acid groups have a stabilizing effect on the benzene ring and make it, therefore, not easy to degrade. In term of toxicity, TA has not been found to be severely toxic to methanogens at the usual concentration of TA present in PTA wastewater [4,27,28]. Also, the partition coefficient (log OW k) of TA has been reported to be 1.16 to 2.00 (depending on which isomer of TA and of the state of the isomer-dis- sociated or non-dissociated) [28]. 3. THERMODYNAMICS OF ANAEROBIC DEGRADATION OF PTA WASTEWATER The main conversion reactions that occur during an- aerobic treatment of PTA wastewater are TA decarboxy- lation, benzoate oxidation, benzoate reduction, and/or carbocyclohexane oxidation. Under the prevalent condi- tions (pH, concentrations and temperature of the waste- water), the TA decarboxylation can proceed but close to the biological limit of –20 KJ·mol–1. The benzoate oxi- dation, however, does not proceed under these condi- tions. The only reaction that is probably promoted is the TA carboxylation (but only for a limited time; until the benzoate concentrations reached a high value). The ben- zoate reduction proceeds because H2 that is needed for the reaction must come from the benzoate oxidation,  M. O. Daramola et al. / Natural Science 3 (2011) 371-378 Copyright © 2011 SciRes. OPEN ACCESS 373 which cannot proceed under these conditions. At pH of 7, however, all the reactions can proceed except the carbo- cyclohexane reaction. Furthermore, the TA decarboxylation reaction might not proceed, because when the TA decarboxylation and benzoate reduction and oxidation are carried out by the same organism, benzoate reduction and oxidation path- way is favoured. This occurs because those two reac- tions deliver more energy than the TA decarboxylation. Figure 1 and Figure 2 depict the different Gibbs free energies for the reactions at the actual concentrations of TA, benzoate and acetate at different pH, 37˚C and 55˚C, respectively (The values of Gibbs free energy of forma- tion of the compounds were taken from Reference [26] and Reference [29]). Therefore, to remove the TA, the benzoate and acetate concentrations should be low. Comparing Figure 1 and Figure 2 shows that an in- crease in temperature from 37˚C to 55˚C improves the energy yield for almost all reactions except the benzoate reduction (see Figure 2). This indicates that higher tem- peratures are preferable for TA degradation. However, Figure 1. Gibbs free energy for the different reactions at dif- ferent pH at the actual concentrations of TA, Benzoate and acetate at 37˚C. Figure 2. Gibbs free energy for the different reactions at dif- ferent pHs at the actual concentrations of TA, benzoate and acetate at 55˚C. Kleerebezem and Lettinga [30] reported unsuccessful results on anaerobic degradation PTA wastewater at thermophilic condition. The failure could be attributed to the possibility of low number of thermophilic anaerobic TA degrading microorganisms in the inoculum’s sludge and/or non-optimal operating conditions during the TA degradation. In the contrary, Chen et al. [31] reported relatively high TA and benzoate removal in one reactor at thermophilic condition compared to the removal at mesophilic condition (0.7 versus 0.5 g TA/gVSS (day)−1). Success of Chen et al. could be attributed to the different TA biodegradation kinetics during thermophilic condi- tion compared to the kinetics during mesophilic condi- tion. At higher temperatures, Gibbs free energies for TA biodegradation are more negative. Thus favouring and enhancing thermophilic PTA degradation. At the same time, the pH should be taken into consideration. Consid- ering the influence of pH, plots on Figures 1 and 2 are only valid for a pH ≥ 5.5 because at pH < 5.5, TA is mainly available in solid form [4]. Also, optimal growth of the TA degrading organisms occurs in a range of pH 6 to 7 [4]. Therefore for feasible anaerobic degradation of the PTA wastewater described in this study (the waste- water has a pH = 5), the pH of the wastewater should be increased to 6 or 7 by adding alkali. In the optimal pH growth range, the benzoate oxidation and reduction gain more energy than the TA decarboxylation. Also, it is likely that only TA decarboxylation will proceed if the benzoate concentration is low as suggested by Kleere- bezem et al. [26]. The hypothesis of Kleerebezem et al. [26] was verified in this study through computations. Based on these computations, we concluded that anaero- bic degradation of the wastewater (our case study) is possible and feasible under both mesophilic and thermo- philic conditions. However, in a case where the pH of the wastewater ≤ 5, during the treatment the pH of the sludge should be increased to 7 (the optimum pH for the anaerobic treatment [30]). Addition of appropriate amount of chemicals such as sodium hydroxide solution (NaOH) sodium bicarbonate (NaHCO3) to the wastewa- ter bioreactor could raise the pH to 7. 4. MICROBIAL POPULATION FOR ANAEROBIC DEGRADATION OF PTA WASTEWATER Identification of structure and diversity of a microbial community is important in degradation processes be- cause it is useful for better understanding of the physio- logical roles of different species in degradation proc- esses. Different types of bacteria have different roles under different conditions. Like in thermophilic and me- sophilic conditions, microbial community that will be  M. O. Daramola et al. / Natural Science 3 (2011) 371-378 Copyright © 2011 SciRes. OPEN ACCESS 374 involved in anaerobic degradation of PTA wastewater will definitely exhibit different roles at those conditions. For mesophilic condition, a temperature range of 35˚C - 37˚C is necessary while for thermophilic degradation, temperature of about 55˚C is essential [32]. In the case study under consideration in this paper, the PTA waste- water is generated at 54˚C - 60˚C, therefore, thermo- philic treatment is preferable. In addition, through the application of thermophilic treatment, the use of cooling units required to cool the PTA wastewater from 60˚C to 37˚C, which require addi- tional costs, could be avoided. According to van Lier et al. [33], thermophilic methanogenic consortia have, often, a higher specific organic removal rate than the mesophilic consortia. However, it is difficult, but important to get the thermophilic organisms in the original inoculum and to provide the required conditions for thermophilic deg- radation [31]. Under mesophilic condition, δ-Proteoba- cteria and the subcluster Ih of the group “Desulfoto- maculum lineage I” [34] have been identified as the mi- croorganisms responsible for the PTA wastewater deg- radation under mesophilic condition. In addition, the syntrophic methanogenic counterparts identified as Me- thanosaeta concilii and members of Methanospirillum and Methanobacteriaceae are the microorganisms which play a role in the degradation process. Some methods like Restriction Fragment Length Poly- morphism (RFLP), Clone libraries and Fluorescence In- Situ Hybridization (FISH) and Scanning Electron Mi- croscopy (SEM) analysis are used to identify microbial population and diversity in anaerobic degradation of PTA wastewater during thermophilic condition. Accord- ing to results from a SEM analysis, six different domi- nant morphotypes have been identified to play signifi- cant roles in degradation process of PTA wastewater under thermophilic anaerobic degradation condition [31]. According to the authors, the dominant cells, the fat rods, are responsible for TA degradation in the reactor. The second most dominant cells, the bamboo-shaped cells, and rods with flat ends (Methanosaeta-like species) are methanogens that utilize acetate. In clone library analysis, according to the investiga- tion on phylotype. Methanothrix thermophila in the ace- toclastic Methanosaeta group is found as the dominant phylotypes. The remaining clones are related to mainly hydrogen-utilizing Methanospirillum species [35]. The Desulfotomaculum group is found to be the most domi- nant that include diverse isolates and clone sequences from various thermophilic and mesophilic environments [36]. The desulfotomaculum group is also identified as a dominant group according to the results of RFLP tech- nique [31]. The FISH results with rRNA-targeted probes were used to identify the domains Archaea (ARC915) and Bacteria (EUB338_I/II/III). According to the results of Loy et al. [37], the Desulfotomaculum group was identi- fied as a dominant group with probe DFMI227a. Under a microscopic examination, the authors observed that these groups referred to the fat rods, are probably responsible for TA degradation under thermophilic conditions. An- other new probe TA55_OP5 was used to identify a dif- ferent population. This probe hybridized to very small rod shape cells, which have the second largest fraction in the sludge. As a result of all methods used to analyze the microbial community, the microbial diversity for ther- mophilic degradation can be specified as Methanothrix thermophila-related methanogens, Desulfotomaculum- -related bacterial populations in the Gram-positive low-G+C group and OP5-related populations, which are responsible for degradation of terephthalate. 5. KINETICS OF PTA WASTEWATER DEGRADATION AND REACTOR DESIGN Kinetics of the system is essential to determining the volume and hydraulic retention time (HRT) of the reac- tor in both mesophilic and thermophilic conditions. In order to study the kinetics of degradation, in many cases, some assumptions are made due to insufficient data from literature. To justify and validate these assumptions, ex- periments are carried out to obtain the kinetic parameters. In the scenario presented in this paper, loading rate of about 10 kg COD m−3·day−1 [26] for mesophilic condi- tion and 16 kg COD m−3·day−1 [31] for thermophilic condition were assumed for the reactor. For mesophilic and thermophilic conditions, the solid (sludge) retention time (SRT) was assumed to be 40 days based on the re- ports presented in Reference [26] and Reference [31]. The authors also reported hydraulic retention time (HRT) of 5 to 8 h in their studies. The operating conditions adap- ted in the computation of HRT, the reactor volume and the chemical oxygen demand (COD) content per m3 wastewater for the scenario presented in this study are presented in Table 2. The HRT obtained was obtained using Eq.1: RTV Q (1) where HRT is the hydraulic retention time (h); V, the bioreactor volume in m3 and Q, the flow rate in m3·h−1. The computed HRT was between 15 and 24 h. This val- ue is higher than HRT reported in Reference [26] and Reference [31] despite using the same SRT. The dis- crepancy can be attributed to the assumptions made in this study. Furthermore, it has been understood that the SRT could be high because of the low growth rate of the  M. O. Daramola et al. / Natural Science 3 (2011) 371-378 Copyright © 2011 SciRes. OPEN ACCESS 375 Table 2. Data used for bioreactor design. Wastewater characteristic Thermophilic condition Mesophilic condition COD (kg COD m−3) 10.25 10.25 Maximum loading rate (kg COD m−3·day−1) 16 10 Solid Retention Time (SRT), day 40 40 PTA degrading bacteria. Therefore, based on the com- puted SRT, volume of bioreactor required for the degra- dation was calculated. The value obtained for the COD was 10.4 kg COD m−3. By considering different effluent flow rates from the production plant, the total loading rate, in kg COD/day, was computed. By dividing the flow rate with the maxi- mal loading rate gives the minimum reactor volume re- quired for the PTA wastewater degradation. For meso- philic condition, the minimum reactor volume required is ca. 1 m3 per 1 m3 wastewater flow per day and for thermophilic condition, ca. 0.6 m3 reactor volume per 1 m3 wastewater flow per day is required. Although, the reactor volume required for PTA wastewater degradation depends on the wastewater flow rate from the production plant. Having considered this, attempt was made to compute reactor volume at different wastewater flow rates for both mesophilic and thermophilic conditions. Figure 3 depicts the reactor volume as a function of the flow rate. From the reactor volume, the HRT was com- puted using Eq.1. Minimum HRT of 24.6 h and 15.4 h were obtained for mesophilic condition and thermophilic condition, respectively: As it can be seen in Figure 3, smaller reactor volume is required for thermophilic anaerobic degradation com- pared to mesophilic anaerobic degradation to treat the same volume of PTA wastewater. This finding is cor- roborated by the studies of Kleerbezem et al. [26] and Chen et al. [31] where it has been shown experimentally that the TA conversion rate is higher in the thermophilic compared to mesophilic. 6. CONCLUSIONS AND FUTURE OUTLOOK Processes involved in the anaerobic treatment of TA wastewater have been discussed from the perspective a case study. A good choice to treat the PTA wastewater, as presented in this case study, is through thermophilic anaerobic degradation reactor. To degrade the amount of the PTA wastewater, one-stage anaerobic reactor will be required for thermophilic condition while at mesophilic condition degradation two-stage reactor is required. How- ever in both cases, and as suggested by Noyola et al. Figure 3. Reactor volume as a function of flow rate. [38], anaerobic degradation alone could not accomplish effective PTA wastewater treatment because the sus- pended solids in the raw wastewater could cause clog- ging and accumulation of toxicity in the reactor [39]. Therefore, it is expected that post-treatment with an aerobic unit could ensure the effluent quality below the international waste disposal benchmark. However, a big fluctuation in the removal efficiency of the reactor also could pose a problem [31]. Thermophilic condition is preferable to mesophilic condition, because with a one-stage reactor at thermo- philic condition, the same removal rate can be achieved as with a two-stage reactor at mesophilic condition. The costs for a two-stage reactor are much higher than for a one-stage process, so one-stage will be preferable. Also, for the fact that the PTA wastewater stream exists at a temperature of 54˚C - 60˚C, cooling units will be re- quired to reduce the temperature before mesophilic deg- radation is feasible. This eventually translates into addi- tional cost for the treatment of the PTA wastewater. But in thermophilic degradation, no cooling unit is required. Moreover, in both cases, the pH of the wastewater should be raised to 6 - 7 by adding alkali because the microbial degrading kinetics is at optimum at this condi- tion. Regarding the future perspective of anaerobic biodeg- radation of PTA wastewater, in-depth investigation of anaerobic degradation mechanism and bio-kinetics is essential. Although, Farjdo et al. [27] reported an inves- tigation on anaerobic degradation mechanism and bio- kinetics using upflow anaerobic sludge blanket (UASB) reactor for easily biodegradable compounds, viz., acetic, benzoic and formic acids from PTA wastewater, however, more research efforts are still needed, especially, for PTA wastewater treatment. As suggested by Karthik et al. [1], pretreatment of PTA wastewater with coagulation-floc- culation prior to biodegradation is techno-economically 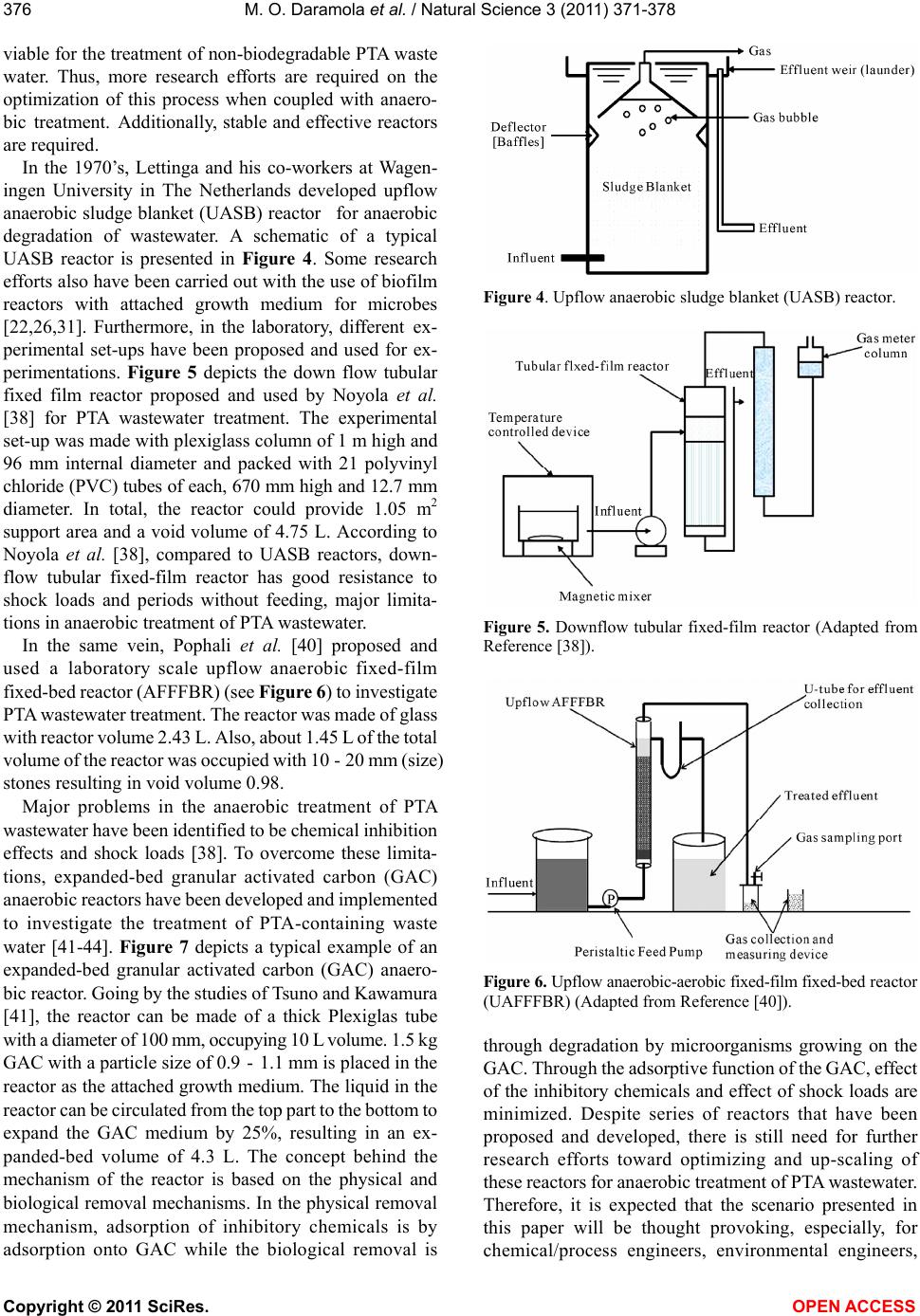 M. O. Daramola et al. / Natural Science 3 (2011) 371-378 Copyright © 2011 SciRes. OPEN ACCESS 376 viable for the treatment of non-biodegradable PTA waste water. Thus, more research efforts are required on the optimization of this process when coupled with anaero- bic treatment. Additionally, stable and effective reactors are required. In the 1970’s, Lettinga and his co-workers at Wagen- ingen University in The Netherlands developed upflow anaerobic sludge blanket (UASB) reactor for anaerobic degradation of wastewater. A schematic of a typical UASB reactor is presented in Figure 4. Some research efforts also have been carried out with the use of biofilm reactors with attached growth medium for microbes [22,26,31]. Furthermore, in the laboratory, different ex- perimental set-ups have been proposed and used for ex- perimentations. Figure 5 depicts the down flow tubular fixed film reactor proposed and used by Noyola et al. [38] for PTA wastewater treatment. The experimental set-up was made with plexiglass column of 1 m high and 96 mm internal diameter and packed with 21 polyvinyl chloride (PVC) tubes of each, 670 mm high and 12.7 mm diameter. In total, the reactor could provide 1.05 m2 support area and a void volume of 4.75 L. According to Noyola et al. [38], compared to UASB reactors, down- flow tubular fixed-film reactor has good resistance to shock loads and periods without feeding, major limita- tions in anaerobic treatment of PTA wastewater. In the same vein, Pophali et al. [40] proposed and used a laboratory scale upflow anaerobic fixed-film fixed-bed reactor (AFFFBR) (see Figure 6) to investigate PTA wastewater treatment. The reactor was made of glass with reactor volume 2.43 L. Also, about 1.45 L of the total volume of the reactor was occupied with 10 - 20 mm (size) stones resulting in void volume 0.98. Major problems in the anaerobic treatment of PTA wastewater have been identified to be chemical inhibition effects and shock loads [38]. To overcome these limita- tions, expanded-bed granular activated carbon (GAC) anaerobic reactors have been developed and implemented to investigate the treatment of PTA-containing waste water [41-44]. Figure 7 depicts a typical example of an expanded-bed granular activated carbon (GAC) anaero- bic reactor. Going by the studies of Tsuno and Kawamura [41], the reactor can be made of a thick Plexiglas tube with a diameter of 100 mm, occupying 10 L volume. 1.5 kg GAC with a particle size of 0.9 - 1.1 mm is placed in the reactor as the attached growth medium. The liquid in the reactor can be circulated from the top part to the bottom to expand the GAC medium by 25%, resulting in an ex- panded-bed volume of 4.3 L. The concept behind the mechanism of the reactor is based on the physical and biological removal mechanisms. In the physical removal mechanism, adsorption of inhibitory chemicals is by adsorption onto GAC while the biological removal is Figure 4. Upflow anaerobic sludge blanket (UASB) reactor. Figure 5. Downflow tubular fixed-film reactor (Adapted from Reference [38]). Figure 6. Upflow anaerobic-aerobic fixed-film fixed-bed reactor (UAFFFBR) (Adapted from Reference [40]). through degradation by microorganisms growing on the GAC. Through the adsorptive function of the GAC, effect of the inhibitory chemicals and effect of shock loads are minimized. Despite series of reactors that have been proposed and developed, there is still need for further research efforts toward optimizing and up-scaling of these reactors for anaerobic treatment of PTA wastewater. Therefore, it is expected that the scenario presented in this paper will be thought provoking, especially, for chemical/process engineers, environmental engineers,  M. O. Daramola et al. / Natural Science 3 (2011) 371-378 Copyright © 2011 SciRes. OPEN ACCESS 377 Figure 7. An expanded-bed granular activated carbon (GAC) anaerobic reactors (Adapted from Reference [41]). scientists and other professionals in environmental ma- nagement, particularly in the area of industrial wastewa- ter treatment. In addition, application of the process di- agnosed in this paper is possible in the treatment of other petrochemical wastewater such as, phenol-containing wastewater, benzene/benzoic acid-containing wastewater or wastewater from other similar industrial settings. 7. ACKNOWLEDGEMENTS MOD is grateful to the authority of Obafemi Awolowo University, Nigeria, for study leave to carry out this study. REFERENCES [1] Karthik, M., Dafale, N., Pathe, P. and Nandy, T. (2008) Biodegradability enhancement of purified terephthalic acid wastewater by coagulation-flocculation process as pre- treatment. Journal of Hazardous Materials, 154, 721-730. doi:10.1016/j.jhazmat.2007.10.085 [2] Chinese Pure Terephthalic Acid Market Report (2007) http://studio-5.financialcontent.com/financialvisions?Cha nnelID=3191&GUID=3460172& Page=MediaViewer2008 [3] Franck, H.G. and Stadelhofer, J.W. (1988) p-Xylene and its derivates: Terephthalic acid. In: Franck, H.G. Ed., In- dustrial Aromatic Chemistry: Raw Materials, Process Products, Springer-Verlag, Berlin, 283-290. [4] Kleerbezem, R., Mortier, J., Hulshoff Pol, L.W. and Let- tinga, G. (1997) Anaerobic pretreatment of petrochemical effluents: Terephthalic acid wastewater. Water Science and Technology, 36, 237-248. doi:10.1016/S0273-1223(97)00393-4 [5] Macarie, H. and Guyot, J.P. (1992) Inhibition of the me- thanogenic fermentation of ptoluic acid (4-methyl-ben- zoic acid) by acetate. Applied Microbiology and Bio- technology, 38, 398-402. doi:10.1007/BF00170093 [6] Liangming, X., Yuxiang, C. and Xiangdong, Z. (1991) The anaerobic biological treatment of high strength pet- rochemical wastewater by a hybrid reactor. International Conference on Petroleum Refining and Petrochemical Processing, Beijing, 11-15 September 1991, 120-126. [7] Kleerebezem, R., Hulshoff Pol, L.W. and Lettinga, G. (1999) Anaerobic biodegradability of phthalic acid iso- mers and related compounds. Biodegradation, 10, 63-73. doi:10.1023/A:1008321015498 [8] Kleerebezem, R., Hulshoff Pol, L.W. and Lettinga, G. (1999) Anaerobic degradation of phthalate isomers by methanogenic consortia. Applied Microbiology and Bio- technology, 65, 1152-1160. [9] Wolkowski, T.R., Chin, T.Y. and Heck, H. (1982) Chemical urolithiasis 3: Pharmacokinetics and transpla- cental transport of terephthalic acid in Fischer-344 rats. Drug Metabolism and Disposition, 10, 486-490. [10] Pernille, E., Kristian, K., Brandt, A., Rasmussen, L.H., Ovesen, R.G. and Sørensen, J. (2007) Microbial degrada- tion and impact of Bracken toxin ptaquiloside on micro- bial communities in soil. Chemosphere, 67, 202-209. doi:10.1016/j.chemosphere.2006.08.025 [11] Qi, S.T., Wang, X.R. and Xu, X.K. (2002) Study on the bladder calculi and bladder cancer induced by terephthalic acid in rats. Journal of hygiene research, 31, 10-12. [12] Scholz, N. (2003) Ecotoxicity and biodegradation of phthalate monoesters. Chemosphere, 53, 921-926. doi:10.1016/S0045-6535(03)00668-4 [13] Chen, B.J., Yuan, Y.X. and Qu, K.M. (2000) Joint effects of acetaldehyde, p-phthalic acid and ethylene glycol on growth of Tetradesmus wiseconsinense. Journal of Fish- ery Sciences of China, 7, 82-85. [14] Qu, K.M., Yuan, Y.X. and Chen, M.S. (2000) Acute tox- icity and join effect of pollutants in fiber wastewater in Daphnia magna. Journal of Fishery Sciences of China, 7, 78-81. [15] Chen, B.J., Yuan, Y.X. and Wang, H.P. (2001) Joint ef- fects of acetaldehyde, p-phthalic acid and ethylene glycol on growth of silver carp and grass carp. Journal of Fish- ery Sciences of China, 8, 73-76. [16] Zhang, H.D., Xu, X.K. and Gong, N. (2001) Study on the toxicity of terephthalic acid to NIH3T3 cells. Chinese Journal of Industrial Medicine, 14, 65-67. [17] US Environmental Protection Agency (2007) Federal Register. http://www.deq.state.va.us/vpdes/pdf/BactiFinalRule.pdf [18] Deng, Y., Zhang, K., Chen, H., Wu, T., Krzyaniak, M., Wellons, A., Bolla, D., Douglas, K. and Zuo, Y. (2006) Iron-catalyzed photochemical transformation of benzoic acid in atmospheric liquids: Product identification and reaction mechanisms. Atmospheric Environment, 40, 3665- 3676. doi:10.1016/j.atmosenv.2006.03.019 [19] Chan, A.H., Chan, C.K., Barford, J.P. and Porter, J.F. (2003) Solar photocatalytic thin film cascade reactor for treatment of benzoic acid containing wastewater. Water Research, 37, 1125-1135. doi:10.1016/S0043-1354(02)00465-7 [20] Brillas, E., Cabot, P.L., Rodriguez, R.M., Arias, C., Gar- rido, J.A. and Oliver, R. (2004) Degradation of the herbi-  M. O. Daramola et al. / Natural Science 3 (2011) 371-378 Copyright © 2011 SciRes. OPEN ACCESS 378 cide 2, 4-DP by catalyzed ozonation using the O3/Fe2+/ UVA system. Applied Catalysis, 51, 117-127. doi:10.1016/j.apcatb.2004.02.007 [21] Radoiu, M.T., Martin, D.I., Calinescu, I. and Iovu, H. (2004) Preparation of polyelectrolytes for wastewater treatment. Journal of Hazardous Materials, 106, 27-37. doi:10.1016/j.jhazmat.2003.08.014 [22] Kleerbezem, R., Beckers, J., Hulshoff Pol, L.W. and Lettinga, G. (2005) High rate treatment of terephthalic acid production wastewater in a two stage anaerobic bio- reactor. Biotechnology and Bioengineering, 91, 169-179. doi:10.1002/bit.20502 [23] Lau, C.M., (1978) Staging aeration for high efficiency treatment of aromatic acids plant wastewater. Proceed- ings of 32nd Independent Wastewater Conference, West Lafayette, 10 May 1977, 63-74. [24] Macarie, H. and Guyot, J.P. (1995) Use of ferrous sul- phate to reduce the redox-potential and allow the start-up of UASB-reactors treating slowly biodegradable com- pounds: Application to a wastewater containing 4-methy- lbenzoic acid. Environmental Technology, 16, 1185-1192. doi:10.1080/09593331608616354 [25] Macarie, H., Noyola, A. and Guyot, J.P. (1992) Anaero- bic treatment of a petrochemical wastewater from a terephthalic acid plant. Water Science and Technology, 25, 223-235. [26] Kleerebezem, R., Pol, L.W.H. and Lettinga, G. (1999) Energetic of product formation during anaerobic degra- dation of phthalate isomers and benzoate. FEMS Micro- biology Ecology, 29, 273-282. doi:10.1111/j.1574-6941.1999.tb00618.x [27] Fajardo, C., Guyot, J.P., Macarie, H. and Monroy, O. (1997) Inhibition of anaerobic digestion by terephthalic acid and its aromatic byproducts. Water Science and Technology, 36, 83-90. doi:10.1016/S0273-1223(97)00510-6 [28] OECD SIDS (2001) Terephthalic acid. UNEP publica- tions, Nairobi. [29] Amend, J.P. and Shock, E.L. (2001) Energetics of overall metabolic reactions of thermophilic and hyperthermo- philic Archaea and Bacteria. FEMS Microbiology Re- views, 25, 175-243. doi:10.1111/j.1574-6976.2001.tb00576.x [30] Kleerebezem, R. and Lettinga, G. (2000) High-rate an- aerobic treatment of purified terephthalic acid wastewater. Water Science and Technology, 42, 259-268. [31] Chen, C.-L., Macarie, H., Ramirez, I., Olmos, A., Ong, S.L., Monroy, O. and Liu, W.T. (2004) Microbial com- munity structure in a thermophilic anaerobic hybrid reactor degrading terephthalate. Microbiology, 150, 3429-3440. doi:10.1099/mic.0.27193-0 [32] Macarie, H. (2000) Overview of the application of an- aerobic treatment to chemical and petrochemical waste- waters. Water Science and Technology, 42, 201-214. [33] Van Lier, J.B., Rebac, S. and Lettinga, G. (1997) High- ate anaerobic wastewater treatment under psychrophilic and thermphilic conditions. Water Science and Technol- ogy, 35, 199-206. doi:10.1016/S0273-1223(97)00202-3 [34] Qiu, Y.L., Sekiguchi, Y., Imachi, H., Kamagata, Y., Tseng, I.C., Ohashi, S.S. and Harada, H. (2004) Identification and isolation of anaerobic, syntrophic phthalate iso- mer-degrading microbes from methanogenic sludges treat- ing wastewater from terephthalate manufacturing. Ap- plied Microbiology and Biotechnology, 70, 1617-1626. [35] Wu, J.H., Liu, W.T., Tseng, I.C. and Cheng, S.S. (2001) Characterization of microbial consortia in an anaerobic granular sludge system treating terephthalate. Microbi- ology, 147, 373-382. [36] Castro, H.F., Williams, N.H. and Ogram, A. (2000) Phy- logeny of sulphate-reducing bacteria. FEMS Microbiol- ogy Ecology, 31, 1-9. [37] Loy, A., Lehner, A., Lee, N., Adamczyk, J., Meier, H., Ernst, J., Schleifer, K.H. and Wagner, M. (2002) Oli- gonucleotide microarray for 16S rRNA gene-based de- tection of all recognized lineages of sulfate-reducing pro- karyotes in the environment. Applied and Environmental Microbiology, 68, 5064-5081. doi:10.1128/AEM.68.10.5064-5081.2002 [38] Noyola, A., Macarie, H. and Guyot, J.P. (1990) Treat- ment of TA plant wastewater with an anaerobic fixed film reactor. Environmental Technology, 11, 239-248. doi:10.1080/09593339009384862 [39] Guyot, J.P., Macarie, H. and Noyola, A. (1990) Anaero- bic digestion of a petrochemical wastewater using the UASB process. Applied Biochemistry and Biotechnology, 24/25, 579-589. doi:10.1007/BF02920280 [40] Pophali, G.R., Khan, R., Dhodapkar, R.S., Nandy, T. and Devotta, S. (2007) Anaerobic-aerobic treatment of PTA effluent: A techno-economic alternative to two-stage aero- bic process. Journal of Environmental Management, 85, 1024-1033. doi:10.1016/j.jenvman.2006.11.016 [41] Tsuno, H. and Kawamura, M. (2009) Development of an expanded-bed GAC reactor for anaerobic treatment of terephthalate – containing wastewater. Water Research, 43, 417-422. doi:10.1016/j.watres.2008.10.046 [42] Suidan, M.T., Strubler, C.E., Kao, S.W. and Pfeffer, J.T. (1983) Treatment of coal gasification wastewater with anaerobic filter technology. Journal of Environmental Management, 55, 1263-1270. [43] Tsuno, H., Kawamura, M. and Somiya, I. (1996) Anaero- bic degradation of pentachlorophenol (PCP) in biological expanded-bed reactor. Water Science and Technology, 34, 335-344. doi:10.1016/0273-1223(96)00663-4 [44] Tsuno, H., Kawamura, M. and Oya, T. (2006) Applica- tion of biological activated carbon anaerobic reactor for treatment of hazardous chemicals. Water Science and Technology, 53, 251-260. doi:10.2166/wst.2006.360
|