Open Journal of Yangtze Oil and Gas
Vol.02 No.03(2017), Article ID:77629,7 pages
10.4236/ojogas.2017.23012
Determination of Heavy Metals and Physicochemical Parameters of Crude Oil Polluted Soil from Ohaji Egbema Local Government in Imo State
Ihenetu Stanley Chukwuemeka1, Ihenetu Francis Chukwuebuka2, Kalu Georgina Ijeoma1
1Chemistry Department, Imo State University, Owerri, Nigeria
2Microbiology Department, Federal University of Technology, Owerri, Nigeria

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 10, 2017; Accepted: July 11, 2017; Published: July 14, 2017
ABSTRACT
The soil physicochemical parameter in Ohaji in Imo State, Nigeria was investigated using Ibeocha, Ekeugba, Awara, Mbirichi and Location as a case study. The result obtained from the soil analysis using standard methods shows that the concentrations of the heavy metals, Pb and Fe were highest in the soil from Ibeocha (0.045 ppm) and Mbirichi (0.126 ppm) which show high rate of industrial activity and oil spill in those areas. There was no remarkable change in pH, but temperature followed the pattern of heavy metal concentration with acidic (lowest) values at the centre. The cation exchange capacity of the soil sample from location (14.066 ppm) shows that this soil sample has a lower contamination to compare to other soil samples from other location. Containment of the effects should be encouraged as soil from these sampling locations is contaminated due to oil spill.
Keywords:
Physiochemical Properties, Soil, Heavy Metals

1. Introduction
Soil is considered to be the skin of the earth and interfaces with its lithosphere, hydrosphere, atmosphere and biosphere [1] . Soil consists of a solid phase (minerals and organic matter) as well as a porous phase that holds gases and water [1] . Accordingly, soils are often treated as a three-state system.
Most soils have a density between 1 g/cm3 and 2 g/cm3. Little of the soil of planet Earth is older than the Pleistocene and none is older than the Cenozoic, although fossilized soils are preserved from as far back as the Archean [2] .
Significance of the Study
This study will go a long way to help determine the heavy metal concentration and other physiochemical characteristics of crude oil polluted soil obtained from Ohaji-Egbema in Imo state, Nigeria.
The aim and objective of this work are to assess the level of the selected heavy metals in soil and to determine the physiochemical parameters of polluted soil samples.
2. Materials and Methods
This research work was carried out among five different oil areas in Ohaji-Eg- bema local government area in Imo State. Ohaji-Egbema covers an area of approximately 958.010 km2 and it liesin the south/western part of Imo State and shares common boundaries with Owerri in the East [3] .
2.1. Sample Collection
Five different Soil samples (Figure 1) were collected in Ohaji-Egbema community, Imo state. Soil samples were collected at different distances at a depth of 15~30 cm using an auger and kept in sterile plastic bags. Particle size composition was obtained by hydrometer method [4] . Each sample was labeled appropriately and transported to the chemical laboratory of the Imo state University Owerri, Nigeria, for analysis.
Figure 1. Geographical location of the site of sampling locations.
2.2. Sample Reduction
Sample reduction was performed by quartering the sample to a convenient amount for analysis. The mixed composite sample were spread on clean plastic to form an even layer. The plastic was marked into quarters and the two opposite quarters are taken and mixed. The process is repeated until the two quarters selected give the desired sample size.
2.3. Preparation and Treatment of Samples for Analysis
Soil samples were air-dried and sieved with a 2 mm mesh [5] . The temperature of each sample was taken at the site by immersing the bulb of the thermometer in the soil and the reading in ˚C taken after one minute.
2.4. Determination of Moisture Content
10 g of the sample was weighed into a porcelain dish. It was placed on a hot air oven at 105˚C for two hours until all the moisture is driven off. The difference in weight is the amount of moisture in the soil. The moisture in the soil is calculated using the formula
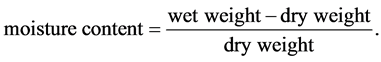
2.5. Determination of Soil Texture
100 g of the sample was weighed into the quart bottle and filled with water up to the neck of the bottle. It was Shake to make sure that they settle simultaneously on the bottom of the quart bottle. It was allowed to stand overnight and the measure of the sand, clay and silt was calculated in percent.
2.6. Determination of Electrical Conductivity
The electrical conductivity was determined by using the HANNA HI8733 electrical conductivity. The electrical conductivity meter was with potassium chloride (KCl). 20 g of the sample was weighed into 500 ml beaker and 200 ml of water was added. It was allowed to stay for about 30 minutes and the electrode of the electrical conductivity was diped into the beaker and the reading was taking.
2.7. Determination of Organic Matter
This was determined by using the muffle furnace. 10 g of the sample was poured it into a porcelain dish and it was placed on a porcelain dish inside a muffle furnace and the temperature was set at 420˚C for 2 hours. The sample was weighed using the electrical weighing balance to check the organic matter. Formular for calculating the organic matter.
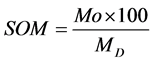
where: Mo is organic matter, MD is mass of dey soil, MA is mass of burned soil.
Mo = MD − MA, MD = Y2 − WD (dry soil from the moisture content) MA = MPA − WD.
2.8. Determination of Soil Temperature
This was determined by using the gardener’s thermometer. The gardener’s thermometer was inserted inside the soil sample and it was allowed to stay for about 5 minutes, the temperature of the soil was then checked.
2.9. Reaction with Hydrochloric Acid
10 g of the sample was poured into a beaker and it was diluted with hydrochloric acid and distilled water in the ratio of 1:3. One drop of the diluted hydrochloric acid was dropped on the sample to check the reaction.
2.10. Determination of the Soil pH
This is done by the use of JENWAY 3510 pH meter which is calibrated using buffer 4 and buffer 7.10 g of the sample was weighed and pour into 250 ml beaker and 100 ml of water was added and stirred for about 30 minutes for 2 hours. The pH electrode was dipped into the beaker containing the sample and water and was left to stay for 5 minutes before taking the reading.
2.11. Determination of Heavy Metals
Heavy metal analysis was conducted using Varian AA240 Atomic Absorption Spectrophotometer according to the method of APHA 1995 (American public health association). 1 g of each sample was digested with aqua-regia for 5 days. The extract was centrifuged 30,000 for 15 mins and the heavy metal was determined using approparite calibration curves prepared in the same acid matrix with standard metal solutions for atomic absorption spectrophotometer
3. Results
The soil’s characteristics that were used as indicators of the levels of pollution are presented in Tables 1-4. Properties like Electrical conductivity, moisture content, pH, organic carbon, heavy metals, cation exchange capacity and visual tests were conducted and their results are shown in the various tables below.
Table 1. Visual characteristics.
where: PW = pinkish white, RB = reddish brown, LRB = light reddish brown, HC = hydrocarbon, Deg. = degradation, E con = evidence of contamination, RW. HCl = reaction with HCl.
Table 2. Physicochemical characteristics.
where: EC = electrical conductivity (µS/cm), MC = moisture content, OM = organic matter, t = temperature, SD = standard deviation, DP = depth of sampling. Values are means of three replicates.
Table 3. Cation exchange capacity.
Where: Ca = calcium, mg = magnesium, K = potassium, Na = sodium, CEC = cation exchange capacity, Ppm = part per million.
Table 4. Concentration of heavy metals.
Where: Pb (ppm) = lead, Fe (ppm) = iron, Ppm = part per million. RSD = relative standard deviation.
4. Discussions
The summary of the result in Table 1 shows that all the soils bears the characteristics of oil soil, starting Ibeocha to Location. From the results obtained from Table 2, the Ibeocha sample recorded the highest electrical conductivity (212 µs/cm) and there is no much difference in the pH and temperature of the samples from the different locations, this is in line with the study made by Ololade et al., 2010, as seen in Table 5. The cation exchange capacity of the soil sample from location (14.066 ppm) is obtained to be higher than other soil samples (5.560~11.057 ppm), from Ibeocha, Ekeugba, Awara and Mbirichi. The cation exchange capacity as seen in Table 3. is high except for Na (ppm) which is not high in some of the sampling locations. The result obtained from Table 4 shows that there is no concentration of Pb in Ekeugba sample (0.00 ppm) but the concentration of Pb in Ibeocha sample (0.045 ppm) is higher than other concentrations of Pb from other sample locations. From Table 4, the concentration of Fe of the sample obtained from Mbirichi community (0.126 ppm) is very high to compare to the concentration of Fe obtained from other sampling points. Figure 2 shows the bar chart indicating the level of Cation exchange capacity for all the samples. Some of these parameter were compared to a research done by Ololade et al. [8] .
Table 5. Physiochemical properties of non-polluted soil in Edo State [6] [7] .
Figure 2. Bar chart showing the rate of Cation exchange capacity (CEC) in all samples.
5. Conclusion
From the analysis done so far, it can be concluded that the five samples obtained from Ibeocha community, Ekeugba community, Awara community, Mbirichi community and Location are contaminated. Therefore, in order to minimize the rate of spills in these areas (community), the following recommendations are suggested. The government of Nigeria should muster the political will to exact stricter respect for environmental laws and regulations by oil companies and a penalty plan established that requires oil companies whose activities cause excessive pollution or are ill-equipped to forfeit their licenses. Multinational and indigenous oil companies should ensure regular and constant inspection and maintenance of oil facilities to avoid accidental discharge or spillage of oil and other petroleum products. The current compensation regime in Nigeria has to be reviewed for it to be fair and adequate to meet the emergency needs and concern of those affected by pollution.
Cite this paper
Chukwuemeka, I.S., Chukwuebuka, I.F. and Ijeoma, K.G. (2017) Determination of Heavy Metals and Physicochemical Parameters of Crude Oil Polluted Soil from Ohaji Egbema Local Government in Imo State. Open Journal of Yangtze Gas and Oil, 2, 161-167. https://doi.org/10.4236/ojogas.2017.23012
References
- 1. White, R.E. (1997) Principles and Practice of Soil Science. 3rd Edition, Blackwell et al Inc., 152.
- 2. Ajithkumar, P.V., Gangadhara, K.P., Manilal, P. and Kunhi, A.A.M. (1998) Soil Inoculation with Pseudomonas aeruginosa 3m T Eliminates the Inhibitory Effect of 3-Chloroand 4-Chlorobenzoate on Tomato Seed Germination. Journal of Soil Biology and biochemistry, 30, 1053.
- 3. Lee, I., Kim, K. and Chang, Y. (2012) Heavy Metal Concentrations and Enzyme Activities in Soil. International Journal of Environmental Research and Public Health, 94, 406-411.
- 4. Doran, J.W. and Parkin, T.B. (1994) Defining and Assessing Soil Quality and Effects of Soil Pollution. New Era Publishers, New York, 7-14.
- 5. Allen, S.E., Grimshaw, P.H., Parkinson, J.A. and Quarmby, C. (1974) Chemical Analysis of Ecological Materials. Blackwell Scientific Publications, London, 41.
- 6. Alex, D.W. (2008) Water Quality Studies of Nworie River in Owerri, Nigeria. Mississippi Academy of Sciences, 10-14.
- 7. Bouyoucos, G.J. (1962) Estimation of Colloidal Material in Soils. Science, 64, 632.
- 8. Ololade, I.A., Ajayi, I.R., Mohammed, O.Z. and Gbadamosi, A.E. (2010) A Study on Effects of Soil Physico-Chemical Properties on Cocoa Production in Ondo State. Journal of Modern Applied Science, 4, 5.




