American Journal of Plant Sciences
Vol.08 No.03(2017), Article ID:73919,14 pages
10.4236/ajps.2017.83025
CO2 and Chamber Effects on Epidermal Development in Field-Grown Peanut (Arachis hypogaea L.)
D. C. Gitz III, J. T. Baker, H. Echevarria-Laza, P. Payton, J. R. Mahan, R. J. Lascano
Cropping Systems Research Laboratory, ARS-USDA*, Lubbock, TX, USA

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 29, 2016; Accepted: February 3, 2017; Published: February 6, 2017
ABSTRACT
Peanut, (Arachis hypogaea L.) cvar. C76-16, was grown either in the field, or in open gas exchange chambers under elevated or ambient CO2 concentrations. Stomatal density and other selected epidermal parameters associated with leaf development and gas exchange were measured on recently fully expanded canopy leaves. It was hypothesized that exclusion of solar UV by chambers would affect stomatal density, but no clear statistically significant chamber effect on stomatal density was found. However, elevated [CO2] did lead to a reduction in both adaxial and abaxial stomatal developmental initiation and in stomatal density. Since each stomate was bounded by companion cells resulting from developmental events, non-random stomatal spacing as the “one cell spacing rule” appears to result from ontogeny rather than a long hypothesized chemical signal inhibiting adjacent meristemoid differentiation into guard cells. A method of visualizing epidermal patterns is also described.
Keywords:
Peanut, Stoma, Stomatal Density, Carbon Dioxide, Climate Change

1. Introduction
The rate, extent, effects, and causes, of global climate change remain contentious issues, especially with regard to the role of anthropogenic greenhouse gas emissions [1] . Regardless of the relative contribution of anthropogenic greenhouse gases to climatic variation, there is unambiguous evidence that a near consistent increase in atmospheric CO2 concentration has occurred over the past half century of monitoring [2] . How crops will respond to increasing atmospheric [CO2], both alone and in conjunction with stressors such as drought and high ambient temperature, continues to be an active area of investigation.
Experiments attempting to examine the effects of increased atmospheric CO2 concentrations on plant growth, development, and physiology, are most commonly conducted within enclosures such as controlled environmental cabinets or greenhouses. While such approaches allow for a comparatively wide range of very finely controlled environmental conditions, they suffer from several disadvantages and so they are seldom used in the field. When such approaches are used in field settings, they typically use permanent or at best unwieldy semipermanent structures [3] . Chamber-less free air CO2 enrichment experiments are less commonly used for a variety of reasons, primarily complexity and cost, but also because other environmental conditions such temperature and rapid microenvironmental fluctuations in CO2 concentration cannot be as finely controlled. Even so, since free air schemes operate in the absence of chambers, they do not introduce micro-environmental effects associated with chambers. This is an advantage because the ultimate test of any ecological or agronomic experiment is the extension of results to natural or field production settings. Successful extension of experimental results to field settings is fraught with the fewer assumptions, the more agronomically realistic the experimental conditions are. From a purely empirical point of view, it has been argued that the vast majority of research conducted in greenhouses and growth chambers exhibits “limited realism for ecological questions” [4] . Nevertheless, from a purely pragmatic point of view, mobile canopy level chamber systems could offer potential advantages such as lower operating costs resulting in the ability to increase CO2 concentrations at night [5] [6] and the ability to more easily better replicate experimental treatments measurements across plots [7] .
A relatively inexpensive field portable open flow Canopy Evapo-Transpiration and Assimilation (CETA) system has been described which allows diurnal gas exchanges to be measured in field grown plants while maintaining air temperatures within 0.5˚C of ambient provided a sufficient canopy size and sufficient soil water to remove excessive latent energy from the system [8] [9] . Later, a CO2 injection system was developed which allowed control of CO2 with precision comparable to that of closed system chambers [10] . These flow-through chambers were constructed of lightweight aluminum frames over which a thin clear polycarbonate film was stretched. Polycarbonate film exhibits a flat spectral transmission of about 95% across the photosynthetically active portion of the spectrum (400 - 700 nm) and has excellent infrared transmission. However, polycarbonate begins to exhibit greatly reduced transmission around 390 nm and is opaque to radiation < 380 nm. Nearly all of the UV-A and UV-B portion of the terrestrial solar spectrum is blocked by the film [11] . While this portion of the terrestrial solar spectrum represents a rather small fraction of the biologically effective solar radiation delivered to plants, the UV portion of the spectrum exerts a disproportionate influence on plant growth and development, especially on epidermal photomorphogenic processes including stomatal differentiation [12] [13] [14] .
The primary objective of this work was to perform a preliminary investigation of potential chamber effects on stomatal density in peanut. A secondary objective was to characterize the effects of elevated CO2 concentration on peanut leaf epidermal development that might influence gas exchange within the CETA chamber system.
2. Materials and Methods
2.1. Plants and Culture
Peanut (Arachis hypogaea L.) cvar. C76?16 seeds from germplasm originally identified by and obtained from Holbrook (15) were planted in the field at the USDA nursery in Lubbock, TX (33˚35'38.9"N, 101˚53'52.1"W, 960 m above sea level) in 2015 on Day of Year (DOY) 148 at a rate of 10,000 seeds ha−1. The C76?16 genotype is a medium to late maturity class runner market (Subspecies hypogaea, alternate branching pattern, flowering restricted to lateral branches, maturity typically 125-145 DAP) breeding line that is considered drought tolerant and pre-harvest aflatoxin contamination resistant [15] . The soil at this location is an Amarillo fine sandy loam (fine-loamy, mixed, superactive, Thermic, Aridic Paleustalf) with a bulk particle size distribution of 75% - 80% sand, 5% - 10% silt, and 10% - 15% clay and an average bulk density of 1.3 g cc3. Seeds were planted at 0.1 m intervals along north-south oriented rows spaced at 1 m. Immediately after planting a timely rainfall delivered 73 mm of water to the plots on DOY 148 and 149. Plants emerged on DOY 160 (>50% emergence) and on DOY 161 locations along the rows were selected and open flow-through CETA cuvettes [8] [9] [10] were placed over 1 m of row. Air was continually moved through the chambers to maintain air temperatures to near that of ambient. The chambers were either fitted with a data logger controlled mass flow controller to maintain 650 µmol (CO2) mol∙L−1 within the chambers (“CETA High”) or without an injection system delivering a flow of un-enriched ambient air (“CETA Ambient”). CO2 treatments began on DOY 173. Water was delivered to the plants over the course of the season by a surface drip irrigation system. The experiment was terminated on DOY 275. Plants grown alongside the CETA cham- bers (“Field Grown”) were also included in this work to provide a preliminary assessment of potential chamber effects on plant development. Local environmental conditions were monitored by a weather station about 300 m west of the plot (http://www.lbk.ars.usda.gov/wewc/weather-pswc-data.aspx) [16] .
2.2. Epidermal Impressions and Image Analysis
Upon termination of the experiment recently fully expanded leaves were collected in the early morning and taken directly to the laboratory located within 50 m from the plots, where they were cleaned and stomatal impressions made by making casts of the epidermal surface as previously described by Gitz and Baker [17] . Fully expanded leaves were defined as the second or third leaf from the shoot apex with length greater than 10 cm. Six leaves were selected from separate plants in one of each chamber treatments (CETA High and CETA Ambient) and four from the Field Grown plants. The leaves were carried to the laboratory, washed in a 1-L beaker containing deionized water with approximately 100 µL of dishwashing detergent added as a surfactant. The leaves were allowed to stand submersed for 5 minutes, gently agitated to loosen debris from the leaf surfaces, rinsed under a stream of deionized water, blotted with paper toweling, and left exposed to the air to allow residual water to evaporate from the surface of the leaves. To remove fibers that could have been transferred to the leaves during blotting, any debris that might have remained after washing, and in an attempt to remove any extra-cuticular waxes or oils that might interfere with subsequent operations, the still-turgid leaves were taken to the fume cupboard where they were washed with a spray of commercial electronic parts cleaner and de-greaser composed of a mixture of tetrachloroethylene and decafluropentane. After the solvent had evaporated over approximately 60 s from the leaf surfaces several coats of a commercial clear nitrocellulose lacquer were applied from an aerosol can allowing each coat to dry to the touch between applications. The lacquer was allowed to harden for in a fume hood and the leaves moved to a clean, smooth bench top where the two distal leaflet pairs were detached from the rachis and carefully arranged side by side on the bench so that the adaxial surface of one and the abaxial surface of the other was exposed. A piece of clear urethane packing tape was placed over the two leaves and gently burnished with a soft cloth to press out entrapped air bubbles and insure intimate contact with the surface of the leaflet. The tape was then removed from the bench surface and the epidermal casts isolated by carefully lifting the petiolules from the tape with fine forceps and then peeling the leaflet away from the epidermal cast, which remained affixed to the tape. The tape with the adhering epidermal casts was then pressed against a 50 mm × 75 mm glass microscope slide, trimmed with a razor edged hobby/utility knife, labeled, and stored until the casts could be viewed.
Digital micrographs of the epidermal casts were captured and used to quantify epidermal features. For stomatal density and stomatal ratio (as Adaxial Stomatal Density/Abaxial Stomatal Density) the glass slide mounted epidermal casts were viewed by bright field microscopy through a 20x objective. Eleven fields were selected from each leaf along a transect parallel to and midway between the midrib and the leaf margin [13] [14] [18] , 640 × 512 pixel images captured, and the images imported into a digital analysis software routine (Sigma Scan, Systat Software Inc., San Jose, CA1) that allowed stoma to be tallied and reduced the possibility of either omitting or counting features multiple times. The image dimensions were determined by capturing images of an “objective” micrometer having 0.01 mm divisions. The area of the images was calculated and the numbers of stoma visible in each image converted to numbers on an area basis (stoma mm−2). The stomatal density of each leaflet was then calculated as the average of the eleven images.
Further quantitation of epidermal features was done through analysis of images acquired from the same epidermal casts, but through a 40× objective (Figure 1). Images from only seven fields of view were collected from each leaflet impression to reduce labor requirements. The images were then resized to twice the original dimensions (from 640 × 512 to 1280 × 1024 pixels), the edges of the guard cells, subsidiary cells, and epidermal tile cells carefully traced with two pixel thick lines (Figure 1(B)), the interior of the objects flooded to generate false color images (Figure 1(C)), and all cells not entirely visible were deleted (Figure 1(D)). After calibrating the image dimensions using the objective micrometer, stomatal lengths, widths, and areas were determined using the image analysis software. Dimensional measurements were made only on completely visible epidermal cells, and completely visible stomates; cells and stomates clipped by the image edge were not included in these measurements (Figure 1(D)).
Numbers of epidermal tile cells and stomata were determined for calculation of stomatal index (Figure 1(C)). Stomatal Index (S.I.) was expressed as:
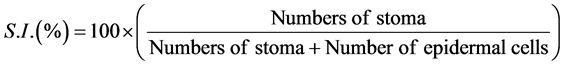
Because each group of subsidiary cells and guard cells arise from a single epidermal meristemoid, stomatal index was also expressed as a Meristem Index
Figure 1. Sequence of operations performed on digital bright field micrographs prior to image analysis. (A) Bright field micrograph taken through 40x objective; (B) Epidermal features traced with 2 pixel wide line; (C) False color image generated by flooding resulting polygons with red (stoma), cyan (subsidiary cells), or yellow (epidermal ground or “tile” cells). All visible cells shown here were counted for cell density related measurements; (D) Features used for dimensional analysis such as area, length of major axis (stoma only), and length of minor axis (stoma only). Only cells not clipped by the image edge were measured. Images were collected through a 40x objective. Bar is 0.1 mm.
(M.I.). Here each stomatal complex refers to all cells arising from a single meristemoid, or “mother cell”, i.e., the guard cells and the neighboring subsidiary cells (Figure 1). The M.I. calculation was identical to S.I. except that stomatal complexes was substituted for numbers of stoma.
2.3. Data Analysis
Separation of means was by multiway t-tests with Bonferroni correction in SAS (19). Only the two preplanned comparisons, Field Grown vs. CETA Ambient and CETA Ambient vs. CETA High are reported. The experimental unit was the plant. One recently expanded canopy leaf was taken from six separate plants in the chamber treatments, or from four plants in the field grown treatments. There were six replicates in the “CETA High” and “CETA Ambient” treatments and four replicates in the “Field Grown” peanut treatment.
3. Results
Selected bright field micrographs of epidermal casts produced from abaxial surfaces of peanut leaves allowed to develop under the three treatments are shown in Figure 2. The total stomatal density, that is, the numbers of stoma on both the adaxial and abaxial surfaces per square millimeter of leaf are shown in the lower right inset of Figure 2 to aid in visualization of the magnitude of the differences illustrated by the selected micrographs.
While reporting data in two formats is generally considered redundant, the total stomatal density results were also included in Table 1 in the interest of
Figure 2. Selected bright field micrographs of epidermal castings taken from abaxial peanut leaf surfaces grown (A) in the field at ambient [CO2], (B) in a CETA chamber at ambient [CO2] and (C) in a CETA chamber at elevated [CO2]. Images were captured through 20× objective. Bars are 0.1 mm. Inset at lower right shows total (adaxial + abaxial) stomatal density as numbers of stoma mm−2 (bars are standard error).
Table 1. Selected morphometric parameters of peanut leaf surfaces as affected by growth and development inside or outside of CETA chambers, and at ambient or elevated CO2 concentrations within CETA chambers. Standard errors (se) are shown in parentheses. Pt < 0.05 are shown in bold italics.
completeness, where the effects of [CO2] and of CETA cuvettes on the other morphological parameters are shown.
Mean stomatal density at ambient CO2 was slightly increased by growth within chambers but this effect was insignificant (Pt > 0.15). No chamber effect on either S.I. or M.I. was found. Chamber effects on epidermal cell numbers and size were restricted to the adaxial surfaces. Stomatal density, M.I., S.I., epidermal cell size, and epidermal cell density of plants within chambers were all affected by CO2. Chamber effects on ambient CO2 grown plants were largely restricted to the adaxial surfaces while CO2 effects on chamber grown plants were evident on both leaf surfaces (Table 1).
The measured stomatal areas averaged from each leaf surface (n = 32) were additionally compared to areas calculated as ellipses with major and minor axes corresponding to measured lengths and widths of stoma (Figure 3). Stomatal size, as measured areas are also compared to areas calculated as rectangles with widths assumed to be one half that of length (20 and references therein). Figure 3 shows results of linear regression and 99% confidence intervals about the fitted line. The fitted linear equation is not plotted for the elliptical model because this resulted in the confidence intervals being very nearly overlain upon it. A near 1:1 agreement was observed with the elliptical model. Linear regression underestimated measured stomatal area by 15% with the rectangular model (slope = 0.83). Standard error of the estimate was an order of magnitude greater for the rectangular model (1.26e−5 mm2) as compared to that of the elliptical model (1.68e−6 mm2).
4. Discussion
The effects of increased [CO2] on growth, development, yield, and water use efficiency in several species of plants, especially crop plants, has been the subject of considerable work over the past quarter century or so. The results of this work have been summarized in periodic reviews [19] [20] [21] . While a number of such studies have been done with peanut in chambers and greenhouses [22] [23] [24] [25] , fewer have been done with field grown plants. In the current study the developing plants were planted in rows as in conventional cropping systems. Clear cuvettes opaque to the UV portion of the terrestrial solar spectrum were placed over the rows and CO2 levels carefully controlled. Roots were not confined as greenhouse container grown plants would be, so the predominate experimental effects were limited to those directly surrounding the aerial parts of the plants. While results from container grown plants have been extended to infer
Figure 3. Measured area of individual stomates vs. area of stoma calculated assuming a regular ellipse with measured major and minor axes (solid lines filled circles), or as rectangles having a width of 1/2 of stomatal length (dotted lines, open squares). Results of linear regression ± 99% confidence intervals are shown.
how plants will respond to elevated [CO2] in the field, it was empirically unknown how peanut plants would respond under CETA chambers in which the roots were unconfined. It was also unknown how the increased light intensity and altered spectral quality, specifically the elimination of most of the ultraviolet radiation, might affect the epidermal photomorphogenic processes. We also questioned how the spectral quality might have affected the sensitivity of leaf developmental responses to increased [CO2].
It could be argued that the experimental design used was actually an incomplete two way design (Figure 2) since there were two factors (inside or outside CETA cuvettes) and two levels (ambient and elevated CO2) which might interact. Stomatal density data were additionally analyzed using both a one-way analysis of variance (ANOVA) with post hoc means separation by the Tukey-Kramer method for unequal sample sizes [26] using the “mixed” model set [27] . However, the results of these analyses did not contribute to the interpretation of the data other than to slightly raise the significance of the observed differences (not shown). For this reason, and because we were originally interested in examining two separate effects rather than the interactions of the two factors it was decided to continue with the more robust multi-way t-test as originally considered. Too, it was thought that the multi-way approach would be fraught with fewer assumptions associated with unpreplanned comparisons.
From a physiological point of view, stomatal pore dimensions and frequency are arguably the most important measurable anatomical parameters with regard to gas exchanges through leaf epidermal surfaces [13] [14] [28] . Stomatal density was determined from images collected at a lower magnification (20× objective) than other parameters (40× objective). So, stomatal counts were collected from fields of view about four times the area of the dimensional measurements. Mean stomatal density calculated from images collected at the higher magnification was slightly larger, about 115%, than the values determined from the lower magnification images (not shown). Likewise variability as standard errors were around 160% that of lower magnification data. Since stoma partially visible at the edge of the images were included in these counts, these differences were attributed, in part, to differences in the image area to image perimeter ratio (lower magnification images result in a perimeter increase of 2× and an area increase of 4×). The increased variability was attributed to the smaller numbers of fields collected for a leaflet and stomatal patchiness. Larger areas (at smaller magnification) effectively averaged four fields of view with less edge error. Although the characterization of epidermal features using the methods reported here required minimal investment, it was time and labor intensive. Because of this, understanding the factors contributing to sampling error and means separation is an important consideration. We suggest that simple density measurements are better done at lower magnifications encompassing more expansive surface areas while morphometric analyses of surface features be done at higher magnifications.
Attempts to relate altered stomatal architecture to gas exchanges are complicated by sample variability [29] [30] . In addition to characterizing the morphological response of the epidermal features, we investigated a potential source of error in estimates of stomatal size. We found that approximating stomatal size (mm2) to be that of a rectangle having length (L) of the major axis of the stomate and a width assumed to be (L/2) to be a remarkably good assumption [29] . However, the results were considerably more variable than an approach less dependent upon assumption (Figure 3). Estimates of stomatal pore depth and maximal diameter that rely on such assumptions might contribute to variability in gas conductivity estimates based upon leaf anatomy, especially between species (e.g. [29] , and references therein). Morphometrically based analyses attempting to estimate the magnitude and significance of potential gas exchange responses to selective breeding and to atmospheric CO2 might consider such variability sources.
The stomatal ratios reported herein are nearly identical to those reported by other workers and stomatal and epidermal cell densities are comparable for plants at the later growth stages [22] . Differences between these two studies may have resulted from different cultivars, growth conditions, and methodology. There may also have been differences in our interpretation of surface features (Figure 1) as compared to other workers (see [22] , esp. Plate 1; [31] ). It should also be borne in mind that attempting to compare the preliminary results presented herein to those obtained from other controlled environmental studies is potentially complicated by the differences in light intensity. The location of the present study was within a semi-arid region with a high number of clear sky days in the growing season [16] . Depending upon location within chambers, peak photosynthetically active radiation (PAR) intensities in sunlit chambers are typically 93% - 105% of ambient, about 1800 - 2100 µmol∙m−2, considerably higher than PAR typically delivered to plants in artificially lit growth cabinets and greenhouse experiments [32] . It should be recognized here that such effects are strongly dependent upon wall materials, chamber geometry, and the presence of the aluminum frame over which the film was stretched and which can cause brief shading over the course of the day. Even so, it is generally recognized and accepted that ambient PAR strongly affects leaf development including stomatal initiation and that these plants were allowed to develop under agronomically comparable light levels.
Any difference in mean total (adaxial + abaxial) stomatal density between the Field Grown and the Ambient CO2 CETA grown plants was of only modest statistical significance (Pt = 0.26), and the adaxial leaf surface accounted for most of this response (Pt = 0.16). Shorter wavelength blue and UV wavebands have been shown to affect stomatal density in soybean, especially on the adaxial leaf surfaces [13] [14] . It remains possible that sample size was not large enough to allow for clear separation of the means. Objectively it can only be stated that no clear effect of the cuvette material on stomatal density was detected and that if such a response occurred it was insignificant due to sample variability and the relatively small difference in the mean response.
As with the potential chamber effect(s), inspection of Table 1 reveals that the response of stomatal density to CO2 concentration was considerably more pronounced on the adaxial surface. Chamber effect on stomatal size as length, width, and area was restricted to the adaxial leaf surfaces. This could be interesting with respect to the hypothetical amphistomatalization of leaves resulting in reduced stomatal resistance to CO2 diffusion. Amphistomatalization is thought to have resulted from selective breeding of crop plants though there are examples of altered stomatal ratios in response to growth under high light [29] [33] . The idea is that when CO2 becomes limiting within leaves, stomatal density or pore size increases particularly on the adaxial surfaces of leaves as compared to the abaxial surface; the leaves become more amphistomatous. This might lead to interactive chamber x CO2 effects, though we were unable to fully characterize these with the experimental design and this was beyond the immediate scope of this work.
Calculation of stomatal index was originally done to adjust stomatal density for ‘dilution’ by expanding epidermal cells [34] . Today, such measurements are done, in part, to characterize the environmental effects upon stomatal initialization across the developing epidermis. The assumption is that in young developing leaves all epidermal cells are potentially meristematic and have an equal probability of developing into stomatal complexes. Any differences in stomatal index must have resulted from environmental cues that affected the likely-hood that an epidermal cell would develop into a stomate. In the leguminosae generally, and with peanut in particular, subsidiary cells arise from para-mesogenous stomatal development.
The result of the para-mesogenous stomatal developmental process is that the entire stomatal complex, consisting of a pair of guard cells bounded by a two unequally sized subsidiary cells, traces its lineage to a single meristematically active epidermal cell sometimes called a meristemoid ( [31] , and references therein.) Traditionally, calculation of S.I. includes the subsidiary cells as part of the population of epidermal cells [34] . So, in addition to calculating S.I. by traditional means, S.I. was also determined by counting the stomatal complex as a unit since all cells within complexes arose from single meristemoids. The effects of growth within the CETA cuvettes and of elevated [CO2] on both S.I. and M.I. were inconsistent with a short wavelength photomorphogenic effect on initiation of stomatal development [13] [14] . No chamber effect on stomatal initiation as S.I. or M.I. was detected. So, any differences must have resulted from differences in leaf expansion. However, elevated [CO2] did elicit a decrease in both density and stomatal initiation. Since both methods of calculating stomatal initiation separated means at near identical probability levels, either S.I. or M.I. calculations appeared equally adequate for hypothesis testing as described herein. However, when making comparisons between species with differing stomatal ontogenies a M.I. approach might be more appropriate. Finally, we suggest that differences in both how epidermal features are measured and how stomatal indices are calculated could be one reason for high variability observed in meta-analysis of responses of plants to paleoclimatic CO2 variability [30] .
Another result of para-mesogenous stomatal development is that stomata cannot be adjacent and that epidermal tile cells cannot flank guard cells (Figure 1). It has long been hypothesized that developing stoma might release low molecular weight compounds or other signaling molecules that inhibit stomatal development [35] . This hypothesis is used to provide a mechanism for the non-random distribution of stomata across leaf surfaces (If stomatal distribution was random, there would occasionally be adjacent stomata.) In the case of peanut each stomate, consisting of the pore and two guard cells, is bounded by two subsidiary cells as a result of cell lineage (Figure 1). Inhibition of stomatal initiation by low molecular weight signaling molecules from adjacent cells appears to be an un-necessary hypothesis for the observed stomatal patterning, in this case at least.
5. Conclusion
Stomatal density and initiation at ambient [CO2] was unaffected by development within CETA chambers. Elevated [CO2] decreased peanut stomatal density by decreasing stomatal initiation. Stomatal spacing results from ontogeny in peanut and may not involve low molecular weight inhibitors of stomatal differentiation. A limitation of the present work was that the initial question asked may be of limited importance given that ultimately it is stomatal conductance and leaf area that are of most importance to water use, which may not be closely related to stomatal density or size.
Acknowledgements
The assistance of Ryan Mounce with image acquisition and analysis is gratefully acknowledged. This research was funded in part by the USDA-ARS Ogallala Aquifer Program, USDA-AFRI Grant No. 2013-67013-21108, and by USDA Base Research funds.
Cite this paper
Gitz III, D.C., Ba- ker, J.T., Echevarria-Laza, H., Payton, P., Mahan, J.R. and Lascano, R.J. (2017) CO2 and Chamber Effects on Epidermal Development in Field-Grown Peanut (Arachis hypogaea L.). American Journal of Plant Sci- ences, 8, 349-362. https://doi.org/10.4236/ajps.2017.83025
References
- 1. Hoffarth, M.R. and Hodson, G. (2016) Green on the Outside, Red on the Inside: Perceived Environmentalist Threat as a Factor Explaining Political Polarization of Climate Change. Journal of Environmental Psychology, 45, 40-49.
https://doi.org/10.1016/j.jenvp.2015.11.002 - 2. Tans, P.P. (2016) Atmospheric CO2 at Mauna Loa Observatory.
http://www.esrl.noaa.gov/gmd/ccgg/trends/ - 3. Uprety, D.C., Garg, S.C., Bisht, B.S., Maini, H.K., Dwivedi, N., Paswan, G., Raj, A. and Saxena, D.C. (2006) Carbon Dioxide Enrichment Technologies for Crop Response Studies. Journal of Scientific and Industrial Research, 65, 859-866.
http://nopr.niscair.res.in/bitstream/123456789/4949/1/JSIR%2065%2811%29%20859-866.pdf - 4. Caldwell, M.M., Flint, S.D. and Searles, P.S. (1994) Spectral Balance and UV Sensitivity of Soybean: A Field Experiment. Plant Cell and Environment, 17, 267-276.
https://doi.org/10.1111/j.1365-3040.1994.tb00292.x - 5. Bunce, J.A. (2014) CO2 Enrichment at Night Affects the Growth and Yield of Common Beans. Crop Science, 54, 1744-1747.
https://doi.org/10.2135/cropsci2013.12.0803 - 6. Asensio, J.S.R., Rachmilevitch, S. and Bloom, A.J. (2015) Responses of Arabidopsis and Wheat to Rising CO2 Depend on Nitrogen Source and Nighttime CO2 Levels. Plant Physiology, 168, 156-163.
https://doi.org/10.1104/pp.15.00110 - 7. Hurlbert, S.H. (1984) Pseudoreplication and the Design of Ecological Field Experiments. Ecological Monographs, 54, 187-211.
https://doi.org/10.2307/1942661 - 8. Baker, J.T., Van Pelt, S., Gitz, D.C., Payton, P., Lascano, R.J. and McMichael, B. (2009) Canopy Gas Exchange Measurements of Cotton in an Open System. Agronomy Journal, 101, 52-59.
https://doi.org/10.2134/agronj2008.0007x - 9. Baker, J.T., Gitz, D.C. and Lascano, R.J. (2014) Field Evaluation of Open System Chambers for Measuring Whole Canopy Gas Exchanges. Agronomy Journal, 106, 537-544.
https://doi.org/10.2134/agronj2013.0449 - 10. Baker, J.T., Gitz, D.C., Payton, P., Broughton, K.J., Bange, M.P. and Lascano, R.J. (2014) Carbon Dioxide Control in an Open System That Measures Canopy Gas Exchanges. Agronomy Journal, 106, 789-792.
https://doi.org/10.2134/agronj13.0450 - 11. Mazza, C.A., Boccalandro, H.E., Giordano, C.V., Battista, D., Scopel, A.L. and Ballaré, C.L. (2000) Functional Significance and Induction by Solar Radiation of Ultraviolet-Absorbing Sunscreens in Field-Grown Soybean Crops. Plant Physiology, 122, 117-126.
https://doi.org/10.1104/pp.122.1.117 - 12. Gitz, D.C. and Liu-Gitz, L. (2003) How Do UV Photomorphogenic Responses Confer Water Stress Tolerance? Photochemistry and Photobiology, 78, 529-534.
https://doi.org/10.1562/0031-8655(2003)078<0529:HDUPRC>2.0.CO;2 - 13. Gitz, D.C., Liu-Gitz, L., Britz, S.J. and Sullivan, J.H. (2005) Ultraviolet-B Effects on Stomatal Density, Water-Use Efficiency, and Stable Carbon Isotope Discrimination in Four Glasshouse-Grown Soybean (Glycine max) Cultivars. Environmental Experimental Botany, 53, 343-355.
https://doi.org/10.1016/j.envexpbot.2004.04.005 - 14. Gitz, D.C., Britz, S.J. and Sullivan, J.H. (2013) Effect of Ambient UV-B on Stomatal Density, Conductance and Isotope Discrimination in Four Field Grown Soybean (Glycine max (L.) Merr.) Isolines. American Journal of Plant Sciences, 4, 100-108.
https://doi.org/10.4236/ajps.2013.412A3012 - 15. Dang, P.M., Chen, C.Y. and Holbrook, C.C. (2013) Evaluation of Five Peanut (Arachis hypogaea) Genotypes to Identify Drought Responsive Mechanisms Utilising Candidate-Gene Approach. Functional Plant Biology, 40, 1323-1333.
https://doi.org/10.1071/FP13116 - 16. Stout, J.E. (2016) Plant Stress & Water Conservation Meteorological Tower. USDA Agricultural Research Service, Lubbock.
http://www.lbk.ars.usda.gov/wewc/weather-pswc-data.aspx - 17. Gitz, D.C. and Baker, J.T. (2009) Methods for Creating Stomatal Impressions Directly onto Archivable Slides. Agronomy Journal, 101, 232-236.
https://doi.org/10.2134/agronj2008.0143N - 18. Gitz, D.C. (1993) Effect of UV-B Radiation on Photosynthesis and Growth of Two Soybean Cultivars. Master’s Thesis, Miami University, Oxford.
- 19. Lawlor, D.W. and Mitchell, R.A.C. (1991) The Effects of Increasing CO2 on Crop Photosynthesis and Productivity: A Review of Field Studies. Plant Cell and Environment, 14, 807-818.
https://doi.org/10.1111/j.1365-3040.1991.tb01444.x - 20. Idso, K.E. and Idso, S.B. (1994) Plant Responses to Atmospheric CO2 Enrichment in the Face of Environmental Constraints: A Review of the Past 10 Years’ Research. Agricultural and Forest Meteorology, 69, 153-203.
https://doi.org/10.1016/0168-1923(94)90025-6 - 21. Ainsworth, E.A. and Long, S.P. (2005) What Have We Learned from 15 Years of Free-Air CO2 Enrichment (FACE)? A Meta-Analytic Review of the Responses of Photosynthesis, Canopy Properties and Plant Production to Rising CO2. New Phytologist, 165, 351-372.
https://doi.org/10.1111/j.1469-8137.2004.01224.x - 22. Clifford, S.C., Black, C.R., Roberts, J.A., Stronach, I.M., Singleton-Jones, P.R., Mohamed, A.D. and Azam-Ali, S.N. (1995) The Effect of Elevated Atmospheric CO2 and Drought on Stomatal Frequency in Groundnut (Arachis hypogaea (L.)). Journal of Experimental Botany, 46, 847-852.
https://doi.org/10.1093/jxb/46.7.847 - 23. Drake, B.G. and González-Meler, M.A. (1997) More Efficient Plants: A Consequence of Rising Atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology, 48, 609-639.
https://doi.org/10.1146/annurev.arplant.48.1.609 - 24. Prasad, P.V., Boote, K.J., Allen Jr., H.L. and Thomas, J.M. (2003) Super-Optimal Temperatures Are Detrimental to Peanut (Arachis hypogaea L.) Reproductive Processes and Yield at Both Ambient and Elevated Carbon Dioxide. Global Change Biology, 9, 1775-1787.
https://doi.org/10.1046/j.1365-2486.2003.00708.x - 25. Bannayan, M., Tojo Soler, C.M., Garcia y Garcia, A., Guerna, L.C. and Hoogenboom, G. (2009) Interactive Effects of Elevated CO2 and Temperature on Growth and Development of a Short and Long Season Peanut Cultivar. Climatic Change, 93, 389-406.
https://doi.org/10.1007/s10584-008-9510-1 - 26. Kramer, C.Y. (1956) Extension of Multiple Range Tests to Group Means with Unequal Numbers of Replications. Biometrics, 12, 309-310.
https://doi.org/10.2307/3001469 - 27. SAS Institute Inc (2008) SAS/STAT® 9.2 User’s Guide. Cary.
- 28. Nobel, P.S. (2009) Leaves and Fluxes. In: Nobel, P.S., Ed., Physicochemical and Environmental Plant Physiology, 4th Edition, Academic Press, San Diego, 364-437.
https://doi.org/10.1016/b978-0-12-374143-1.00008-9 - 29. Milla, R., de Diego-Vico, N. and Martín-Robles, N. (2013) Shifts in Stomatal Traits Following the Domestication of Plant Species. Journal of Experimental Botany, 64, 3137-3146.
https://doi.org/10.1093/jxb/ert147 - 30. Royer, D.L. (2001) Stomatal Density and Stomatal Index as Indicators of Paleoatmospheric CO2 Concentration. Review of Palaeobotany and Palynology, 114, 1-28.
https://doi.org/10.1016/S0034-6667(00)00074-9 - 31. Farooqui, P. (1982) Ontogeny of the Paracytic Stoma: Variations and Modifications. Proceedings of the Indian Academy of Sciences, 91, 145-152.
- 32. Kim, S.H., Reddy, V.R., Baker, J.T., Gitz, D.C. and Timlin, D.J. (2004) Quantification of Photosynthetically Active Radiation Inside Sunlit Growth Chambers. Agricultural and Forest Meteorology, 126, 117-127.
https://doi.org/10.1016/j.agrformet.2004.06.004 - 33. Mott, K.A. and Michaelson, O. (1991) Amphistomy as an Adaptation to High Light Intensity in Ambrosia cordifolia (Compositae). American Journal of Botany, 78, 76-79.
https://doi.org/10.2307/2445230 - 34. Salisbury, E.J. (1928) On the Causes and Ecological Significance of Stomatal Frequency, with Special Reference to the Woodland Flora. Philosophical Transactions of the Royal Society B: Biological Sciences, 216, 1-65.
https://doi.org/10.1098/rstb.1928.0001 - 35. Willmer, C. and Fricker, M. (1996) The Distribution of Stomata. In: Black, M. and Charlwood, B., Eds., Stomata, Springer, Berlin, Vol. 2, 12-35.
https://doi.org/10.1007/978-94-011-0579-8_2
NOTES

*The US Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who required alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
1Mention of this or other proprietary products is for the convenience of the readers only, and does not constitute endorsement or preferential treatment of these products by USDA-ARS.





