Pharmacology & Pharmacy
Vol.07 No.07(2016), Article ID:69088,11 pages
10.4236/pp.2016.77034
Delivery of Plasmid DNA into Tumors by Intravenous Injection of PEGylated Cationic Lipoplexes into Tumor-Bearing Mice
Yoshiyuki Hattori
Department of Drug Delivery Research, Hoshi University, Tokyo, Japan

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 July 2016; accepted 24 July 2016; published 27 July 2016
ABSTRACT
For systemic injection of cationic liposome/plasmid DNA (pDNA) complexes (cationic lipoplexes), polyethylene glycol (PEG)-modification (PEGylation) of lipoplexes can enhance their systemic stability. In this study, we examined whether intravenous injection of PEGylated cationic lipoplexes into tumor-bearing mice could deliver pDNA into tumor tissues and induce transgene expression. PEGylation of cationic liposomes could prevent their agglutination with erythrocytes. However, when PEGylated cationic lipoplexes were injected intravenously into tumor-bearing mice, they accumulated in tumor vascular vessels and did not exhibit transgene expression in tumors with both poor and well-developed vascularization. Furthermore, PEGylated cationic lipoplexes of CpG- free pDNA could not increase transgene expression in tumors after intravenous injection. These results suggested that PEGylation could not extravasate cationic lipoplexes from vascular vessels in tumors and abolished transgene expression although it enhanced the systemic stability of cationic lipoplexes by avoiding interactions with blood components such as erythrocytes. Successful delivery of pDNA to tumors by PEGylated cationic liposomes will require a rational strategy and the design of liposomal delivery systems to overcome the issue associated with the use of PEG.
Keywords:
Cationic Liposome, Lipoplex, Plasmid DNA, PEGylation, Tumor

1. Introduction
Gene therapy has become an increasingly important strategy for treating a variety of human diseases, including cancer [1] . Cationic liposomes are one of the non-viral vectors for cancer gene delivery [2] . Among cationic liposomes, 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/cholesterol (Chol) [3] [4] , 1,2-di-O-octade- cenyl-3-trimethylammonium propane chloride (DOTMA)/Chol [4] [5] and dimethyldioctadecylammonium bromide (DDAB)/Chol liposomes [6] , have often been used for the transduction of plasmid DNA in vivo. In systemic cancer gene therapy, a therapeutic gene must be delivered efficiently to tumor tissues; however, the positive charge of cationic liposome/plasmid DNA (pDNA) complexes (lipoplexes) leads to interactions with albumin and other serum proteins when injected intravenously. It has been reported that cationic lipoplexes induced gene expression mostly in the lung after intravenous injection [4] . The reason for accumulation of lipoplexes in the lung was agglutination caused by electrostatic interactions between positively charged lipoplexes and negatively charged erythrocytes [7] . The agglutinates contributed to high entrapment of lipoplexes in the highly extended lung capillaries and induced expression predominantly in lung [8] . Therefore, to improve the delivery of cationic lipoplexes to tumors by intravenous administration, the lipoplexes must be stabilized in the blood by avoiding agglutination with blood components.
In systemic injection of cationic lipoplexes, modification of the liposomes with polyethylene glycol (PEG) in the formulation has been reported to enhance systemic stability [9] . PEG modification (PEGylation) on the surface of cationic lipoplexes (PEGylated lipoplexes) can decrease accumulation in the lung and increase accumulation in tumors by avoiding association with blood components. Furthermore, Smrekar et al. reported that gene expression in tumors after intravenous injection of polyethylenimine (PEI)/pDNA complex (PEI polyplex) was critically dependent on the vascularization of the tumor with not only the number and size but also the type of blood vessels [10] . They found that PEI polyplexes could lead to high gene expression in well vascularized tumors, but poorly vascularized tumors showed low gene expression when injected intravenously into tumor-bearing mice. Therefore, in this study, we examined whether PEGylated DOTAP/Chol lipoplex could deliver pDNA into well or poorly vascularized tumors and induce gene expression.
2. Materials and Methods
2.1. Materials
1,2-Dioleoyl-3-trimethylammonium-propane methyl sulfate salt (DOTAP) was obtained from Avanti Polar Lipids Inc. (Alabaster, AL, USA). LissamineTM rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanola- mine, triethylammonium salt (rhodamine-DHPE) was purchased from Invitrogen Co. (Carlsbad, CA, USA). Methoxy-poly(ethyleneglycol)-distearylphosphatidylethanolamine (PEG2000-DSPE, PEG mean molecular weight, 2000) was obtained from NOF Co. Ltd. (Tokyo, Japan). Generation 5 of poly(amidoamine) dendrimer with ethylenediamine core (PAMAM dendrimer G5) was purchased from Sigma-Aldrich (MO, USA). All other chemicals were of the finest grade available.
2.2. Cell Culture
Murine colon adenocarcinoma Colon 26, murine sarcoma S180, and murine Lewis lung carcinoma LLC cells were obtained from the Cell Resource Center for Biomedical Research, Tohoku University (Miyagi, Japan). Murine neuroblastoma Neuro2a were obtained from the European Collection of Cell Cultures (ECACC, Wiltshire, U.K.). Murine lung carcinoma M109 cells were obtained from Division Chemotherapy (Translational Research Center), Chiba Cancer Center (Chiba, Japan).
Colon 26, S180, and LLC cells were cultured in RPMI-1640 medium with 10% heat-inactivated fetal bovine serum (FBS) and kanamycin (100 μg/mL) in a humidified atmosphere containing 5% CO2 at 37˚C. Neuro2a cells were grown in EMEM culture medium supplemented with 10% heat-inactivated FBS and kanamycin (100 μg/mL) at 37˚C in a 5% CO2 humidified atmosphere. M109 cells were subcultured by employing the biogenic system of BALB/c mice (8 weeks of age; Sankyo Lab. Service Corp., Tokyo, Japan).
2.3. Tumor Model
All animal experiments were performed with approval from the Institutional Animal Care and Use Committee of Hoshi University. For generation of Colon 26 and M109 tumors, 1 × 106 cells suspended in 100 μL of phosphate-buffered saline (PBS) were inoculated subcutaneously into the flank of female BALB/c mice (8 weeks of age; Sankyo Lab. Service Corp.), for generation of LLC tumors, into the flank of female C57BL/6Cr mice (8 weeks of age; Sankyo Lab. Service Corp.), for generation of Neuro2a tumors, into the flank of female A/J Jms Slc mice (8 weeks of age; Sankyo Lab. Service Corp.), and for generation of S180 tumors, into the flank of female ddY mice (8 weeks of age; Sankyo Lab. Service Corp.). The tumor volume was calculated using the following formula: tumor volume = 0.5 × a × b2, where a and b are the larger and smaller diameters, respectively.
2.4. Immunohistochemical Analysis
To examine the vascular structure in Colon 26, LLC, M109, Neuro2a, and S180 tumors, their tumors were frozen on dry ice and sliced at 16 μm. Their sections were incubated with rat anti-mouse CD31 (PECAM-1) monoclonal antibody (Clone MEC 13.3, BD Pharmingen, San Diego, CA, USA) for the detection of mouse endothelial cells, and subsequently incubated with goat anti-rat IgG conjugated to Alexa Fluor 488 (Invitrogen) as a secondary antibody. In the detection of mouse pericytes, the sections were further incubated with Cy3-conju- gated rabbit anti-smooth muscle α-actin (α-SMA) antibody (Sigma-Aldrich). Immunofluorescence was examined microscopically using an ECLIPSE TS100-F microscope (Nikon, Tokyo, Japan).
2.5. Preparation of Plasmid DNA
The plasmid pCMV-luc encoding the firefly luciferase gene under the control of the cytomegalovirus (CMV) promoter was constructed as described previously [11] . The plasmid pCpG-free-Luc encoding the firefly luciferase gene under the control of human elongation factor 1 alpha promoter and mouse CMV enhancer was constructed as described previously [12] . pCpG-free-Luc has no CpG motif in the sequence of pDNA; in contrast, pCMV-Luc has CpG motifs. A protein-free preparation of the pDNA was purified after alkaline lysis using an EndoFree Plasmid Max Kit (Qiagen, Hilden, Germany).
2.6. Preparation of Liposomes, Lipoplexes, and Polyplexes
Cationic liposomes were prepared from DOTAP: Chol at a molar ratio of 1:1 by a dry-film method [12] . Briefly, all lipids were dissolved in chloroform, which was removed by evaporation. For preparation of rhodamine-labeled cationic liposomes, rhodamine-DHPE was incorporated into the liposomal formulation at 1 mol% in the total lipid. The thin film was hydrated with water at 60˚C by vortex mixing and sonication. The particle size distributions and ζ-potentials were determined by the dynamic light scattering method (ELS-Z2; Otsuka Electronics, Osaka, Japan) at 25˚C after diluting the dispersion to an appropriate volume with water. Size and ζ-potential of cationic liposomes were 70 nm and +47 mV, respectively.
Cationic liposome/pDNA complexes (cationic lipoplexes) was prepared by mixing pDNA with liposomes at a charge ratio (+:−) of 4:1 with gentle shaking and standing for 15 min at room temperature. For modification of the surface of the lipoplexes by PEG-lipid, the lipoplexes were further incubated with PEG2000-DSPE at 5 mol% in the total lipid at 50˚C for 15 min, and the resulting formulation was allowed to cool to room temperature. Size and ζ-potential of the lipoplexes after PEGylation were 125 nm and +33 mV, respectively.
PAMAM dendrimer G5/pDNA complex (PAMAM polyplexes) were prepared by mixing PAMAM dendrimer G5 with pDNA at a charge ratio (+:−) of 2:1 as reported previously [13] . Size and ζ-potential of PAMAM polyplexes were about 110 nm and +25 mV, respectively.
For preparation of anionic liposomes, the liposomes were prepared from hydrogenated soybean phosphatidylcholine (HSPC):Chol:rhodamine-DHPE at a molar ratio of 55:45:1 by a dry-film method. Briefly, all lipids were dissolved in chloroform, which was removed by evaporation. The thin film was hydrated with PBS by vortex mixing and sonication. For modification of the surface of the liposomes by PEG-lipid, the liposomes were further incubated with PEG2000-DSPE at 5 mol% in the total lipid at 60˚C for 60 min, and the resulting formulation was allowed to cool to room temperature. Size and ζ-potential of PEGylated anionic liposomes were 140 nm and −40 mV, respectively.
2.7. Luciferase Activity in Vitro
Colon 26, LLC, Neuro2a, and S180 cells were prepared by plating cells in a 6-well plate 24 h prior to each experiment. Cationic lipoplexes with 2 μg of pCMV-luc were transfected into the cells for 24 h. Lipofectamine 2000 lipoplexes (Invitrogen) were transfected in accordance with the manufacturer’s protocol. Twenty-four hours after transfection, luciferase activity was measured as counts per second (cps)/μg protein using the luciferase assay system (PicaGene, Toyo Ink Mfg. Co. Ltd., Tokyo, Japan) and bicinchoninic acid (BCA) reagent as reported previously [14] .
2.8. Agglutination Assay
Agglutination assays were performed in accordance with the method described in a previous report [15] . Erythrocytes were collected from mouse blood at 4˚C by centrifugation at 300 g for 3 min and resuspended in PBS as a 2% (v/v) stock suspension of erythrocytes. The cationic liposomes were added to 100 μL of erythrocyte suspension and then incubated for 15 min at 37˚C. The sample was placed on a glass plate and agglutination was observed using a microscope.
2.9. Biodistribution of PEGylated Lipoplexes and PEGylated Liposomes in Tumor-Bearing Mice
When the average volume of the tumors reached 100 - 200 mm3, rhodamine-labeled cationic lipoplexes with 50 μg of pCMV-Luc were administered intravenously via the lateral tail vein into mice. One hour after injection, fluorescent DNA-binding dye Hoechst 33,342 was injected intravenously at 25 mg/kg for detection of tumor vessels with blood flow, and mice were sacrificed 1 min after the injection. For investigation of biodistribution of PEGylated anionic liposomes, rhodamine-labeled liposomes were administered intravenously via the lateral tail vein into mice. Twenty-four hours after injection, Hoechst 33,342 was injected at 25 mg/kg, and mice were sacrificed 1 min after the injection. The tumors were frozen on dry ice and sliced at 16 μm. The localizations of rhodamine-labeled lipoplexes and Hoechst 33,342 were examined using an Eclipse TS100-F microscope (Nikon, Tokyo, Japan) as described previously [16] .
2.10. Transfection Activity in Vivo
When the average volume of the tumors reached 100 - 200 mm3, cationic lipoplexes with 50 μg of pCMV-Luc or pCpG-free-Luc were administered intravenously via the lateral tail vein into Colon 26, LLC, M109, Neuro2a, and S180 tumor-bearing mice. At 24 h post-injection, mice were sacrificed by cervical dislocation, and tissues and tumors were removed for analysis. Three microliters of ice-cold reporter lysis buffer (Promega Co., WI, USA) per 1 mg of tissues or tumors was added, and then homogenized immediately. The homogenate samples were centrifuged at 15,000 rpm for 3 min at 4˚C. Aliquots of 10 μL of the supernatants were mixed with 50 μL luciferase assay system (PicaGene) and counts per second (cps) were measured using a chemoluminometer (Wallac ARVO SX 1420 multilabel counter; Perkin Elmer Life Science, Japan). The protein concentration of the supernatants was determined using a BCA protein assay (Microplate BCA Protein Assay Kit-Reducing Agent Compatible; Pierce, Rockford, IL, USA) with bovine serum albumin as the standard, and luciferase activity was calculated as cps/mg protein.
3. Results and Discussion
3.1. Vascular Structure of Subcutaneous Tumors
Most solid tumors have blood vessels with a defective architecture, such as large gaps between endothelial cells and lack of smooth muscle layers, so that macromolecules such as liposomes will have the opportunity to escape from tumor blood vessels and accumulate selectively in tumor tissues (enhanced permeability and retention [EPR] effect). Therefore, utilization of the EPR effect is an effective strategy for delivery of liposomes to the site of a tumor. However, tumor microenvironments including vascularity, permeability, or blood flow are different among different types of tumors [10] .
First, we investigated the morphological differences among subcutaneously implanted Colon 26, LLC, M109, Neuro2a, and S180 tumors. Major morphological differences were found between the different tumor models at macroscopic levels. M109 and Neuro2a tumors showed an intense red color and blood-filled areas, indicating well developed vascularization (Figure 1(a)). In contrast, the macroscopic view of dissected LLC, Colon 26, and S180 tumors showed a white or pale color, indicating poor vascularization.
Next, we characterized the vascular structure of tumors by immunohistochemical analysis. Examination of Neuro 2a and S180 tumor sections showed that large open vessels were well covered with α-SMA-positive pericytes, but narrow vessels were not (Figure 1(b)), indicating that the vasculature of Neuro 2a and S180 tumors
Figure 1. Gross appearances (a) and immunostaining for endothelial cells and pericytes (b) of subcutaneously implanted tumors. In (a), dissected Colon 26, LLC, M109, Neuro2a, and S180 tumors are shown. In (b), green signals indicate CD31-positive endothelial cells and red signals anti-smooth muscle α-actin-positive pericytes. Scale bar = 100 µm.
might be relatively stable and non-leaky. In contrast, most of the CD31-positive endothelial cells were not covered with α-SMA-positive pericytes in Colon 26, LLC and M109 tumors, suggested that the vasculature of these tumors might be leaky.
3.2. In Vitro Transfection Efficiency
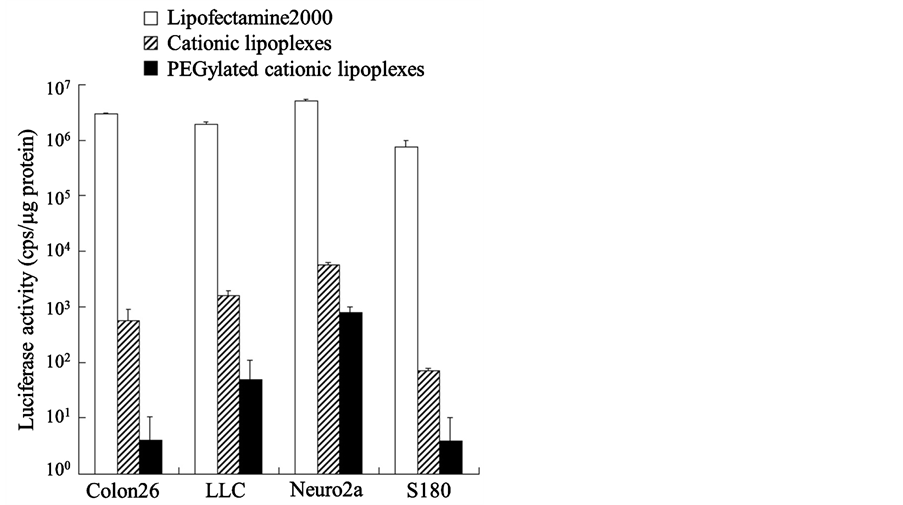
Long circulating liposomes of 100 - 200 nm in size accumulate efficiency in tumors by the EPR effect (passive targeting) [17] . In general, PEGylation of neutral liposomes with approximately 5 mol% PEG-lipid in the total lipid allowed long-term circulation of liposomes in the blood after intravenous administration [18] . Therefore, we prepared PEGylated cationic lipoplexes of pCMV-luc with 5 mol% PEG-lipid, and investigated whether PEGylated cationic lipoplexes could be taken up by tumor cells and induce luciferase expression at 24 h after transfection. However, PEGylation reduced luciferase expression by cationic lipoplexes in Colon 26, LLC, Neuro2a, and S180 cells (Figure 2). These results indicated that PEG coating of the liposomes inhibited the interaction of cationic lipoplexes with tumor cells, and reduced transgene expression. This reduced transgene expression by lipoplexes with PEG are referred to as the PEG dilemma [19] .
3.3. Interaction with Erythrocytes
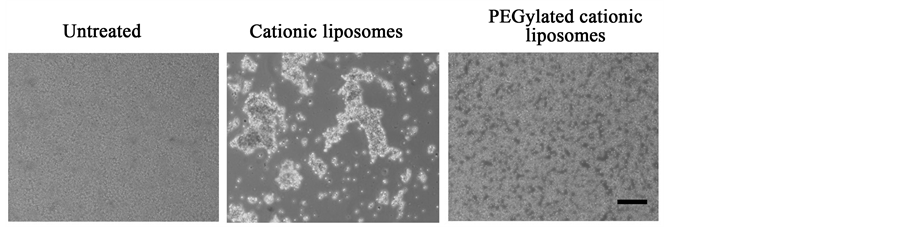
To investigate whether PEG coatings of cationic liposomes could prevent agglutination with erythrocytes, we observed the agglutination of PEGylated cationic liposomes with erythrocytes by microscopy. PEGylated cationic liposomes showed no agglutination, although cationic liposomes did (Figure 3), indicating that PEG coating on the surface sterically hindered the interaction of erythrocytes with the liposomes.
3.4. Luciferase Expression in Vivo in Various Tumor Models
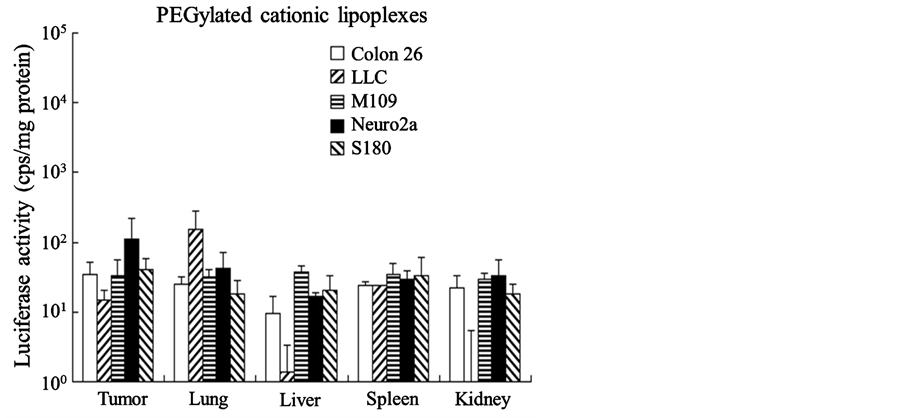
Next, we evaluated the transfection efficiency into mice bearing Colon 26, LLC, M109, Neuro2a, and S180 tumors by assaying luciferase activity 24 h after injection of PEGylated cationic lipoplexes with pCMV-luc. As a result, PEGylated cationic lipoplexes did not exhibit high luciferase activity in any organs, including tumors (Figure 4). A significant difference in luciferase expression levels in tumors was not observed among the tumor models. This finding indicated that PEGylated cationic lipoplexes could not induce transgene expression even in tumors with well-developed vascularization.
Figure 2. Effect of polyethylene glycol-modified (PEGylated) cationic lipoplexes on in vitro gene expression in Colon 26, LLC, Neuro2a, and S180 cells. Luciferase activities were measured at 24 h after transfection of cationic lipoplexes or PEGylated cationic lipoplexes with pCMV-Luc into the cells. Each column represents the mean ± S.D. (n=3).
Figure 3. Agglutination of cationic liposomes with erythrocytes. Cationic liposomes were added to erythrocytes, and then agglutination was observed by phase contrast microscopy. Scale bar = 100 μm.
3.5. Tumor Distribution of PEGylated Cationic Lipoplexes and PEGylated Anionic Liposomes
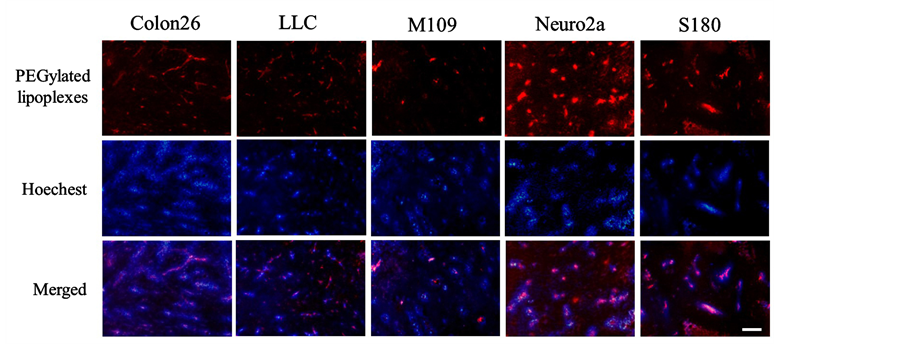
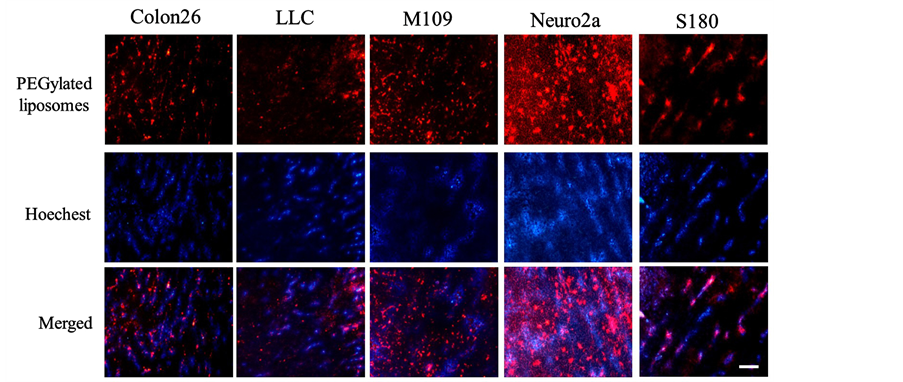
PEGylated cationic lipoplexes are rapidly eliminated from blood circulation after intravenous administration [9] . To investigate whether PEGylated cationic lipoplexes could deliver into tumor tissues after intravenous injection, we examined localization of rhodamine-labeled PEGylated cationic lipoplexes in tumor by fluorescence microscopy at 1 h after intravenous injection into mice bearing Colon 26, LLC, M109, Neuro2a, and S180 tumors (Figure 5). As a result, PEGylated cationic lipoplexes accumulated mainly in the tumor vasculature, indicating that the partially unshielded surface charge of PEGylated cationic lipoplexes might be sufficient to bind to negatively charged tumor vessels [20] although PEGylation of cationic liposomes could abolish interactions with blood components in the circulation.
PEGylation of conventional liposomes has been proved to successfully stabilize these particles and to increase their blood circulation time and passive tumor delivery. Stealth liposomes, which have neutral or negative charge on the surface, are used to deliver doxorubicin efficiency to tumors, and have longer circulating half-life
Figure 4. Luciferase activities at 24 h after intravenous administration of polyethylene glycol-mod- ified (PEGylated) lipoplexes into tumor-bearing mice. PEGylated cationic lipoplexes with 50 μg of pCMV-Luc were administered intravenously via the lateral tail vein into mice bearing Colon 26, LLC, M109, Neuro2a, and S180 tumors. Each column represents the mean ± S.D. (n = 4).
Figure 5. Tumor distribution of polyethylene glycol-modified (PEGylated) cationic liposomes at 1 h after intravenous administration of PEGylated cationic lipoplexes into tumor-bearing mice. Rhodamine-labeled PEGylated cationic lipoplexes with 50 μg of pCMV-Luc were administered intravenously via the lateral tail vein into Colon 26, LLC, M109, Neuro2a, and S180 tumor-bearing mice. Red signals indicate the localization of rhodamine-labeled liposomes, and blue signals show tumor vessels with blood flow. Scale bar = 100 μm.
in blood than PEGylated cationic lipoplexes. Therefore, to examine the effect of surface charge of liposomes on distribution in tumor, we prepared rhodamine-labeled PEGylated anionic liposomes, and examined the localization of PEGylated anionic liposomes in tumors at 24 h after intravenous injection into tumor-bearing mice (Figure 6). As a result, in Neuro2a and M109 tumors, a large number of PEGylated anionic liposomes were obviously extravasated out of the vascular vessels. In contrast, in Colon 26, LLC, and S180 tumors, most of the liposomes were localized within vascular vessels, but some were observed out of the vascular vessels. These findings suggested that PEGylated anionic liposomes could be efficiently delivered to the tumor cells through vascular vessels in M109 and Neuro2a tumors with well-developed vascularization but not in LLC, Colon26, and S180 tumor with poor vascularization. Therefore, to deliver pDNA efficiently into well-vascularized tumors by intravenous injection of lipoplexes, the surface charge of lipoplexes might need to change from positive to negative. These results were consistent with previous reports that cationic liposomes accumulated extensively in tumor
Figure 6. Tumor distribution of rhodamine-labeled polyethylene glycol-modified (PEGylated) anionic liposomes at 24 h after intravenous administration into Colon 26, LLC, M109, Neuro2a, and S180 tumor-bearing mice. Red signals indicate the localization of rhodamine-labeled liposomes, and blue signals show tumor vessels with blood flow. Scale bar = 100 μm.
vessels [20] [21] .
3.6. Luciferase Expression in Vivo by PEGylated Lipoplexes with CpG-Free Plasmid DNA
Bacterial DNA and bacterially derived plasmid DNA have CpG dinucleotides with unmethylated cytosine, which have been identified to be major contributors to the low level and brief transgene expression in vertebrates after non-viral gene delivery [22] . Unmethylated CpG motifs within plasmid DNA are recognized as a pathogen-associated molecular pattern upon acidification of endosomes by the toll-like receptor 9 (TLR-9), which is present at the endosomal membrane [23] . This recognition results in the production of pro-inflamma- tory cytokines [24] , which reduce transgene expression in mice after injection of pDNA with cationic liposomes [25] . CpG motif free plasmid DNA can lead to prolong transgene expression levels in tumors but also other organs such as the lung and liver [26] . Therefore, we compared the transfection efficiencies into mice bearing LLC tumors by assaying luciferase activity 24 h after injections of cationic lipoplexes with pCMV-luc (pDNA with CpG motif) and pCpG-free-Luc (pDNA without CpG motifs), respectively. As a result, cationic lipoplexes with pCpG-free-Luc showed higher luciferase activities in LLC tumors and lung than those with pCMV-Luc (Figure 7(a)). However, PEGylated cationic lipoplexes with pCpG-free-Luc exhibited low luciferase activities in LLC and Neuro2a tumors, compared with cationic lipoplexes without PEGylation (Figure 7(b) and Figure 7(c)).
Navarro et al. reported that PAMAM dendrimer/CpG-free plasmid DNA polyplexes could induce transgene expression in Neuro2a tumor after intravenous injection [13] . Therefore, we confirmed whether injection of PAMAM dendrimer/pCpC-free-Luc polyplexes could induce luciferase activity in tumors. As a result, PAMAM polyplexes showed relatively high luciferase activity in both Neuro2a and LLC tumors (Figure 7(d)), indicating that PAMAM polyplexes might be more suitable for transgene expression in tumors compared with PEGylated cationic lipoplexes.
Many studies have focused on elucidating the optimal concentration of PEG-lipid that should incorporated in the liposomal membrane to provide liposomes with long circulating characteristics. Gjetting et al. reported that 10% PEGylation of DOTAP/Chol lipoplexes reduced retention in lung and heart of mice compared with non-PEGylated lipoplexes, although transgene expression by the lipoplexes was lost upon increasing the degree of PEGylation [9] . Levchenko et al. demonstrated that PEG concentrations of ≥6 mol% shield the electronic surface potential of cationic liposomes [28] . In this study, we showed that cationic lipoplexes modified with 5 mol% PEG2000-DSPE could not induce transgene expression even in tumors with well-developed vascularization. Furthermore, in a preliminary study, we found that modification of cationic lipoplexes with 2 mol% PEG2000- DSPE or PEG350-DSPE did not also induce high transgene expression in any organs when the lipoplexes were intravenously injected into Colon 26 tumor-bearing mice (data not shown). Therefore, in pDNA delivery with PEGylated lipoplexes, the strategies for removing the PEG moiety from PEGylated cationic lipoplexes at the
Figure 7. Effect of CpG motifs in plasmid DNA (pDNA) on gene expression in tumors at 24 h after intravenous administration of cationic lipoplexes or polyplexes into mice bearing LLC or Neuro2a tumors. In A, cationic lipoplexes with 50 μg of pCMV-Luc or pCpG-free-Luc were administered intravenously into LLC tumor-bearing mice. In B, cationic lipoplexes with 50 μg of pCpG-free-Luc were administered intravenously into LLC or Neuro2a tumor-bearing mice. In C, PEGylated cationic lipoplexes with 50 μg of pCpG-free-Luc were administered intravenously into LLC or Neuro2a tumor-bearing mice. In D, PAMAM polyplexes with 50 μg of pCpG-free-Luc were administered intravenously into LLC or Neuro2a tumor-bearing mice. Each column represents the mean ± S.D. (n = 3). Data of luciferase activity by pCpG-free-Luc in LLC tumor-bearing mice in Figure 7(a) and Figure 7(b) were used from reference [27] , and data of luciferase activity by pCpG-free-Luc in Neuro2a-tumor bearing mice in Figure 7(c) was used from reference [12] .
tumor in response to the tumor environment, such as low pH and enzymes expressed in tumors (tumor-specific cleavable PEG system), might improve tumor uptake and transgene expression after intravenous injection of PEGylated lipoplexes.
4. Conclusion
PEGylated DOTAP/Chol lipoplexes after intravenous injection accumulated in tumor vascular vessels and did not induce expression in tumors with good or poor vascularization. Furthermore, PEGylated cationic lipoplexes with CpG-free pDNA could not improve transgene expression in tumors. Successful gene delivery to tumors will require a rational strategy and the design of a liposomal delivery system to overcome the issues associated with the use of PEG. Further improvement of the formulation of cationic liposomes might be required to obtain better transfection in vivo.
Acknowledgements
We thank Ms. Mako Kato, Ms. Mihoko Komori, and Mr. Takumi Tanaka for their assistance with the experimental work.
Conflicts of Interest
The author has no conflict of interest.
Cite this paper
Yoshiyuki Hattori, (2016) Delivery of Plasmid DNA into Tumors by Intravenous Injection of PEGylated Cationic Lipoplexes into Tumor-Bearing Mice. Pharmacology & Pharmacy,07,272-282. doi: 10.4236/pp.2016.77034
References
- 1. Dachs, G.U., Dougherty, G.J., Stratford, I.J. and Chaplin, D.J. (1997) Targeting Gene Therapy to Cancer: A Review. Oncol Res. 9, 313-325.
- 2. Morille, M., Passirani, C., Vonarbourg, A., Clavreul, A. and Benoit, J.P. (2008) Progress in Developing Cationic Vectors for Non-Viral Systemic Gene Therapy against Cancer. Biomaterials, 29, 3477-3496.
http://dx.doi.org/10.1016/j.biomaterials.2008.04.036 - 3. Zhang, Y., Bradshaw-Pierce, E.L., Delille, A., Gustafson, D.L. and Anchordoquy, T.J. (2008) In Vivo Comparative Study of Lipid/DNA Complexes with Different in Vitro Serum Stability: Effects on Biodistribution and Tumor Accumulation. Journal of Pharmaceutical Sciences, 97, 237-250.
http://dx.doi.org/10.1002/jps.21076 - 4. Yeeprae, W., Kawakami, S., Suzuki, S., Yamashita, F. and Hashida, M. (2006) Physicochemical and Pharmacokinetic Characteristics of Cationic Liposomes. Pharmazie, 61, 102-105.
- 5. Sakurai, F., Nishioka, T., Saito, H., Baba, T., Okuda, A., Matsumoto, O., Taga, T., Yamashita, F., Takakura, Y. and Hashida, M. (2001) Interaction between DNA-Cationic Liposome Complexes and Erythrocytes Is an Important Factor in Systemic Gene Transfer via the Intravenous Route in Mice: The Role of the Neutral Helper Lipid. Gene Therapy, 8, 677-686.
http://dx.doi.org/10.1038/sj.gt.3301460 - 6. Hong, K., Zheng, W., Baker, A. and Papahadjopoulos, D. (1997) Stabilization of Cationic Liposome-Plasmid DNA Complexes by Polyamines and Poly(Ethylene Glycol)-Phospholipid Conjugates for Efficient in Vivo Gene Delivery. FEBS Letters, 400, 233-237.
http://dx.doi.org/10.1016/S0014-5793(96)01397-X - 7. Eliyahu, H., Servel, N., Domb, A.J. and Barenholz, Y. (2002) Lipoplex-Induced Hemagglutination: Potential Involvement in Intravenous Gene Delivery. Gene Therapy, 9, 850-858.
- 8. Simberg, D., Weisman, S., Talmon, Y., Faerman, A., Shoshani, T. and Barenholz, Y. (2003) The Role of Organ Vascularization and Lipoplex-Serum Initial Contact in Intravenous Murine Lipofection. Journal of Biological Chemistry, 278, 39858-39865.
http://dx.doi.org/10.1074/jbc.M302232200 - 9. Gjetting, T., Arildsen, N.S., Christensen, C.L., Poulsen, T.T., Roth, J.A., Handlos, V.N. and Poulsen, H.S. (2010) In Vitro and in Vivo Effects of Polyethylene Glycol (PEG)-Modified Lipid in DOTAP/Cholesterol-Mediated Gene Transfection. International Journal of Nanomedicine, 5, 371-383.
- 10. Smrekar, B., Wightman, L., Wolschek, M.F., Lichtenberger, C., Ruzicka, R., Ogris, M., Rodl, W., Kursa, M., Wagner, E. and Kircheis, R. (2003) Tissue-Dependent Factors Affect Gene Delivery to Tumors in Vivo. Gene Therapy, 10, 1079-1088.
http://dx.doi.org/10.1038/sj.gt.3301965 - 11. Igarashi, S., Hattori, Y. and Maitani, Y. (2006) Biosurfactant MEL-A Enhances Cellular Association and Gene Transfection by Cationic Liposome. Journal of Controlled Release, 112, 362-328.
http://dx.doi.org/10.1016/j.jconrel.2006.03.003 - 12. Kato, M., Hattori, Y., Kubo, M. and Maitani, Y. (2012) Collagenase-1 Injection Improved Tumor Distribution and Gene Expression of Cationic Lipoplex. International Journal of Pharmaceutics, 423, 428-434.
http://dx.doi.org/10.1016/j.ijpharm.2011.12.015 - 13. Navarro, G., Maiwald, G., Haase, R., Rogach, A.L., Wagner, E., de Ilarduya, C.T. and Ogris, M. (2010) Low Generation PAMAM Dendrimer and CpG Free Plasmids Allow Targeted and Extended Transgene Expression in Tumors after Systemic Delivery. Journal of Controlled Release, 146, 99-105.
http://dx.doi.org/10.1016/j.jconrel.2010.04.030 - 14. Hattori, Y. and Maitani, Y. (2005) Folate-Linked Nanoparticle-Mediated Suicide Gene Therapy in Human Prostate Cancer and Nasopharyngeal Cancer with Herpes Simplex Virus Thymidine Kinase. Cancer Gene Therapy, 12, 796-809.
http://dx.doi.org/10.1038/sj.cgt.7700844 - 15. Hattori, Y., Nakamura, A., Arai, S., Nishigaki, M., Ohkura, H., Kawano, K., Maitani, Y. and Yonemochi, E. (2014) In Vivo siRNA Delivery System for Targeting to the Liver by Poly-l-Glutamic Acid-Coated Lipoplex. Results in Pharma Sciences, 4, 1-7.
- 16. Hattori, Y., Ubukata, H., Kawano, K. and Maitani, Y. (2011) Angiotensin II-Induced Hypertension Enhanced Therapeutic Efficacy of Liposomal Doxorubicin in Tumor-Bearing Mice. International Journal of Pharmaceutics, 403, 178-184.
http://dx.doi.org/10.1016/j.ijpharm.2010.10.009 - 17. Ishida, O., Maruyama, K., Sasaki, K. and Iwatsuru, M. (1999) Size-Dependent Extravasation and Interstitial Localization of Polyethyleneglycol Liposomes in Solid Tumor-Bearing Mice. International Journal of Pharmaceutics, 190, 49-56.
http://dx.doi.org/10.1016/S0378-5173(99)00256-2 - 18. Papahadjopoulos, D., Allen, T.M., Gabizon, A., Mayhew, E., Matthay, K., Huang, S.K., Lee, K.D., Woodle, M.C., Lasic, D.D., Redemann, C., et al. (1991) Sterically Stabilized Liposomes: Improvements in Pharmacokinetics and Antitumor Therapeutic Efficacy. Proceedings of the National Academy of Sciences of the United States of America, 88, 11460-11464.
http://dx.doi.org/10.1073/pnas.88.24.11460 - 19. Hatakeyama, H., Akita, H. and Harashima, H. (2013) The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of Liposomes for Systemic Genes and Nucleic Acids Delivery to Tumors. Biological and Pharmaceutical Bulletin, 36, 892-899.
http://dx.doi.org/10.1248/bpb.b13-00059 - 20. Krasnici, S., Werner, A., Eichhorn, M.E., Schmitt-Sody, M., Pahernik, S.A., Sauer, B., Schulze, B., Teifel, M., Michaelis, U., Naujoks, K. and Dellian, M. (2003) Effect of the Surface Charge of Liposomes on Their Uptake by Angiogenic Tumor Vessels. International Journal of Cancer, 105, 561-567.
http://dx.doi.org/10.1002/ijc.11108 - 21. Campbell, R.B., Fukumura, D., Brown, E.B., Mazzola, L.M., Izumi, Y., Jain, R.K., Torchilin, V.P. and Munn, L.L. (2002) Cationic Charge Determines the Distribution of Liposomes between the Vascular and Extravascular Compartments of Tumors. Cancer Research, 62, 6831-6836.
- 22. Tousignant, J.D., Zhao, H., Yew, N.S., Cheng, S.H., Eastman, S.J. and Scheule, R.K. (2003) DNA Sequences in Cationic Lipid:pDNA-Mediated Systemic Toxicities. Human Gene Therapy, 14, 203-214.
http://dx.doi.org/10.1089/10430340360535760 - 23. Takeshita, F., Gursel, I., Ishii, K.J., Suzuki, K., Gursel, M. and Klinman, D.M. (2004) Signal Transduction Pathways Mediated by the Interaction of CpG DNA with Toll-Like Receptor 9. Seminars in Immunology, 16, 17-22.
http://dx.doi.org/10.1016/j.smim.2003.10.009 - 24. Yoshida, H., Nishikawa, M., Yasuda, S., Mizuno, Y. and Takakura, Y. (2008) Cellular Activation by Plasmid DNA in Various Macrophages in Primary Culture. Journal of Pharmaceutical Sciences, 97, 4575-4585.
http://dx.doi.org/10.1002/jps.21302 - 25. Tan, Y., Li, S., Pitt, B.R. and Huang, L. (1999) The Inhibitory Role of CpG Immunostimulatory Motifs in Cationic Lipid Vector-Mediated Transgene Expression in Vivo. Human Gene Therapy, 10, 2153-2161.
http://dx.doi.org/10.1089/10430349950017149 - 26. De Wolf, H.K., Johansson, N., Thong, A.T., Snel, C.J., Mastrobattista, E., Hennink, W.E. and Storm, G. (2008) Plasmid CpG Depletion Improves Degree and Duration of Tumor Gene Expression after Intravenous Administration of Polyplexes. Pharmaceutical Research, 25, 1654-1662.
http://dx.doi.org/10.1007/s11095-008-9558-7 - 27. Hattori, Y., Yamasaku, H. and Maitani, Y. (2013) Anionic Polymer-Coated Lipoplex for Safe Gene Delivery into Tumor by Systemic Injection. Journal of Drug Targeting, 21, 639-647.
http://dx.doi.org/10.3109/1061186X.2013.789035 - 28. Levchenko, T.S., Rammohan, R., Lukyanov, A.N., Whiteman, K.R. and Torchilin, V.P. (2002) Liposome Clearance in Mice: The Effect of a Separate and Combined Presence of Surface Charge and Polymer Coating. International Journal of Pharmaceutics, 240, 95-102.
http://dx.doi.org/10.1016/s0378-5173(02)00129-1