American Journal of Analytical Chemistry
Vol. 3 No. 3 (2012) , Article ID: 18261 , 6 pages DOI:10.4236/ajac.2012.33029
Structural, Thermal and Optical Studies of Oxypeucedanin Hydrate Monoacetate Micro-Crystals from Prangos pabularia
1Department of Chemistry, University of Kashmir, Srinagar, J&K-India
2Department of Material Science and Condense Matter Physics, S.N. Bose National Centre for Basic Sciences, Kolkata, India
3Bio-Organic Division, Indian Institute of Integrative Medicine (CSIR), Jammu, J&K-India
4Department of Chemistry, University of Jammu, Jammu, J&K-India
Email: *javidbanday@rediffmail.com
Received December 30, 2011; revised February 6, 2012; accepted February 16, 2012
Keywords: Prangos pabularia; Oxypeucedanin Hydrate Monoaceate; Furanocoumarin; Triclinic; Non-Linear Optical
ABSTRACT
Oxypeucedanin hydrate monoacetate (Mol. Formula = C18H18O7) micro-crystals were obtained by acetylation of oxypeucedanin hydrate, a furanocoumarin, isolated from the roots of Prangos pabularia. The composition related structural, thermal and optical properties were investigated. All the crystals were found to have triclinic structure. From thermal studies of these crystals, stability, melting point and other relevant informations were obtained. Their blue emission, less absorption in entire visible range and band gap predicts these crystals as good candidate for modern optoelectronic devices.
1. Introduction
The current technological trend demands the devotion of interest to identify the organic compounds exhibiting large second-order hyper polarizabilities and their processing for use in non-linear optical (NLO) materials and other optoelectronic devices [1].
In this direction, a lot of advancement has been made in the field of organic electronics, resulting in the demonstration of prototype devices such as a 4.7 inch QVGA (Quarter Video Graphics Array) active matrix display containing 76,800 organic transistors [2], mechanical sensors [3] and chemical sensors [4]. Devices, such as organic light emitting diodes (OLEDs), which are essential part of display technology, are now mostly used in consumer electronics instead of LCD. Most of these have been possible with the availability of a number of organic materials, conducting polymers, insulators, semiconductors and metals. Besides other conventional organic materials, biomaterials are of particular interest. Biomaterials sometimes show strange properties which are not easily replicated in conventional organic or inorganic materials. In addition, natural biomaterials are from renewable resources and are inherently biodegradable. Among natural biodegradable materials, the condense matter community has shown interest in DNA for various reasons, such as potential use of DNA assembly in molecular electronic devices [5], nano-scale robotics [6] and DNA-based computation [7].
The studies about the epitaxial growth of aromatic compounds such as p-nitrophenol [8], p-nitroaniline [9] on PTFE substrates have indicated that the polar axis of the substituted aromatic molecules is oriented along the PTFE chain axis with a very low angular dispersion. The compounds like 2-methyl-4-nitroaniline (MNA) also shows non-centrosymmetric behavior [10]. Nonlinear optical (NLO) materials find extensive optoelectronic applications such as optical frequency conversion, optical data storage and optical switches in the inertially confined laser fusion systems. Of late, second-order nonlinear optical (SONLO) materials, capable of efficient frequency conversion of infrared or visible laser radiation to visible or ultraviolet (UV) wavelengths, are of considerable interest in the field of telecommunications, high density optical recording, color display, medical diagnostics, etc. Therefore, presently there is a need to produce highly efficient NLO materials capable of generating blue light by second harmonic generation [11-14]. Materials with second-order optical nonlinearities, short transparency cut off wavelength, high thermal and mechanical properties are needed in order to realize most of these applications. The nonlinearity of the organic material is increased due to the delocalized π-electron system between donor and acceptor groups, which enhances their asymmetric polarizability [15-17]. In present paper, we report the structural, optical and thermal properties of oxypeucedanin hydrate monoacetate micro-crystals prepared by acetylation of oxypeucedanin hydrate, isolated from the ethyl acetate extract of the root parts of the plant Prangos pabularia.
2. Experimental
Melting points were determined in centigrade scale in one end open capillary on Buchii 570 melting point apparatus and are uncorrected. IR spectrum was recorded on Perkin-Elmer Paragon-1000 spectrophotometer Esquire 3000 spectrometer. 1H and 13C NMR spectra were recorded by a Bruker 500 and 125 MHz instruement using TMS as internal standard and CDCl3 as solvent. UV spectra were recorded on a Shimadzu UV-1601 spectrometer. Column chromatography was carried out with Merk silica gel (60 - 120 mesh and 100 - 200 mesh). The crystals were characterized by x-ray diffraction (XRD) with a Rigaku powder diffractometer for structural study. Photo Luminescence (PL) was carried out by using spectroflorometer by JY-Horiba. Thermo gravimetric analysis (TGA) measurements were carried out on a Perkin Elmer Diamond TA/TGA at a heating rate of 10˚C/min under a nitrogen flow.
3. Results and Discussions
3.1. Structural Analysis
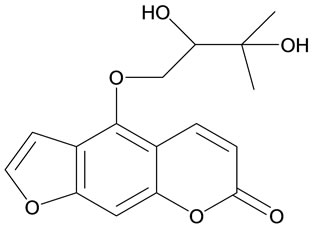
Oxypeucedanin hydrate Figure 1(a), m.p. 134.5˚C, molecular formula C16H16O6 was isolated from ethyl acetate extract of the roots of the plant Prangos pabularia by column chromatography using silica gel as adsorbent. The structure of (a) was confirmed by comparison of its spectral data (1H-NMR, 13C-NMR, UV, IR and MS) with the data values reported in literature [18]. Compound (a) was subjected to acetylation with acetic anhydride and pyridine at room temperature to give compound Figure 1(b), m.p. 138˚C, molecular formula C18H18O7.
On comparison of the spectral data with the data values reported in literature [18], compound (b) was identified as oxypeucedanin hydrate monoacetate.
The Figure 2, shows the powder X-ray diffractogram of oxypeucedanin hydrate monoacetate micro-crystal. From this data, the appearance of sharp peaks at specific Bragg’s angles is clear indication of crystallinity of this
 (a)
(a)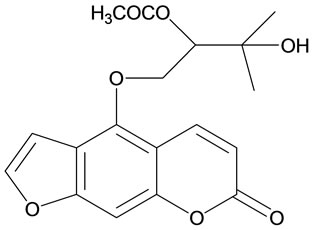 (b)
(b)
Figure 1. Structures of compounds (a) and (b).
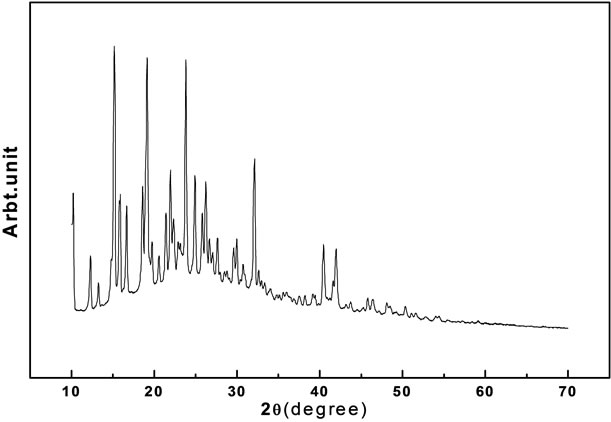
Figure 2. XRD image oxypeucedanin hydrate monoacetate micro-crystal.
system.
The diffractograms were indexed using Powder-X software. Calculation of cell parameters reveals that this crystal belongs to triclinic crystal structure having space group P111. The cell parameters for this crystal are a = 6.22992 Å, b = 7.35151 Å, c = 15.11041 Å, α = 90.821˚, β = 97.330˚ and γ = 113.093˚. The experimental d-values are in conformity with the calculated ones using the above cell parameters for this system. From further analysis, other structural parameters like density and cell volume were calculated. The present observed parameters are well in agreement with other similar systems [19]. The structural parameters are also well correlated with other techniques like NMR and IR carried out on this system. These observations are quite consistent with other reports of the similar system [20].
Furthermore, oxypeucedanin hydrate monoacetate micro-crystal was not of good color and developed with slightly irregular morphology (see Figure 3). These crystals were whitish brown in color without much transparency. The possible reason behind this observation may be the nucleation centers and the ordering of carbon molecules [21].
3.2. Optical Properties
The UV-VIS NIR spectra of oxypeucedanin hydrate monoacetate micro-crystal is shown in Figure 4.
It is clear that the absorbance is less in the wavelength range 350 - 700 nm for these crystals. Here, clearly no absorbance due to electronic transitions in these regions of this system is seen. Also, the possibility of any overtones or combination modes above the threshold values of wavelength for these crystals is not seen. Since, less absorbance in the entire visible region is an essential condition for the Non-linear optical (NLO) applications,

Figure 3. SEM micrograph of oxypeucedanin hydrate monoacetate micro-crystal.

Figure 4. UV-Visible spectra for oxypeucedanin hydrate monoacetate micro-crystal.
good transmittance exhibited by these crystals predicts them good candidate for the generation of second harmonic light (532 nm) as well as third harmonic generation (354.6 nm) for Nd:YAG laser (1064 nm).
Further, the fundamental absorption edge in most semiconductors follows the exponential law. Above the exponential tail, the absorption coefficient of the semiconductor was observed to obey the equation: Some Common Mistakes
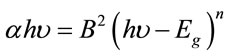 (1) [22]
(1) [22]
where α is the absorption coefficient and υ frequency, B is a constant and n is an index which can be assumed to have values of 1/2, 3/2, 2 and 3, depending on the nature of electronic transition responsible for the absorption, i.e., n = 1/2 for the direct allowed transition (high energy part of the spectra), n = 3/2 for forbidden direct transition, n = 2 for the indirect allowed transition (low energy part of the spectra) and n = 3 for forbidden indirect transition [23]. The variation of (αhυ)n with hυ (Tauc plot), for these crystals, is shown in Figure 5.
The values of Eg for the sample have been calculated by extrapolating the linear region of the curves to meet the hυ axis at (αhυ)n = 0, and are presented in Figure 5. The band-gap of pure crystal prepared by similar method is 4.2 eV [11]. The band-gap in our case, however, appears slightly lesser, which can be attributed to the nature of excitation.
When molecule or compound absorbs light of an appropriate wavelength, an electron is promoted to a higher energy orbital. Also, ultraviolet (UV) and visible (Vis) light has enough energy to cause electronic transition. The higher energy electronic transition is promotion of an electron from a π bonding molecular orbital into a π* antibonding molecular orbital, known as π-π* transition (ligand π-π* transition). This means that only compounds with π electron and non-bonding electrons can produce UV-Vis spectra [15-17]. The observed less absorbance/ high transmittance in the entire visible region is due to π-π* transition of this molecule. This observed high transmittance in these molecules (in visible region) is much higher than that of various other organic molecules, supposed to be useful in NLO devices.
The Photo-luminiscence studies of the oxypeucedanin hydrate monoacetate micro-crystal is presented in Figure 6.
We observe various intensity peaks, but peaks at 404 and 430 nm are dominant ones. The reason for these emissions is not fully known. Possible reason can be C=C bond and π-π* transition of this molecule. However, the exact knowledge is beyond the scope of this paper (study). The presence of these two peaks in visible region gives a clear indication that this material can be used for organic optoelectronic devices.
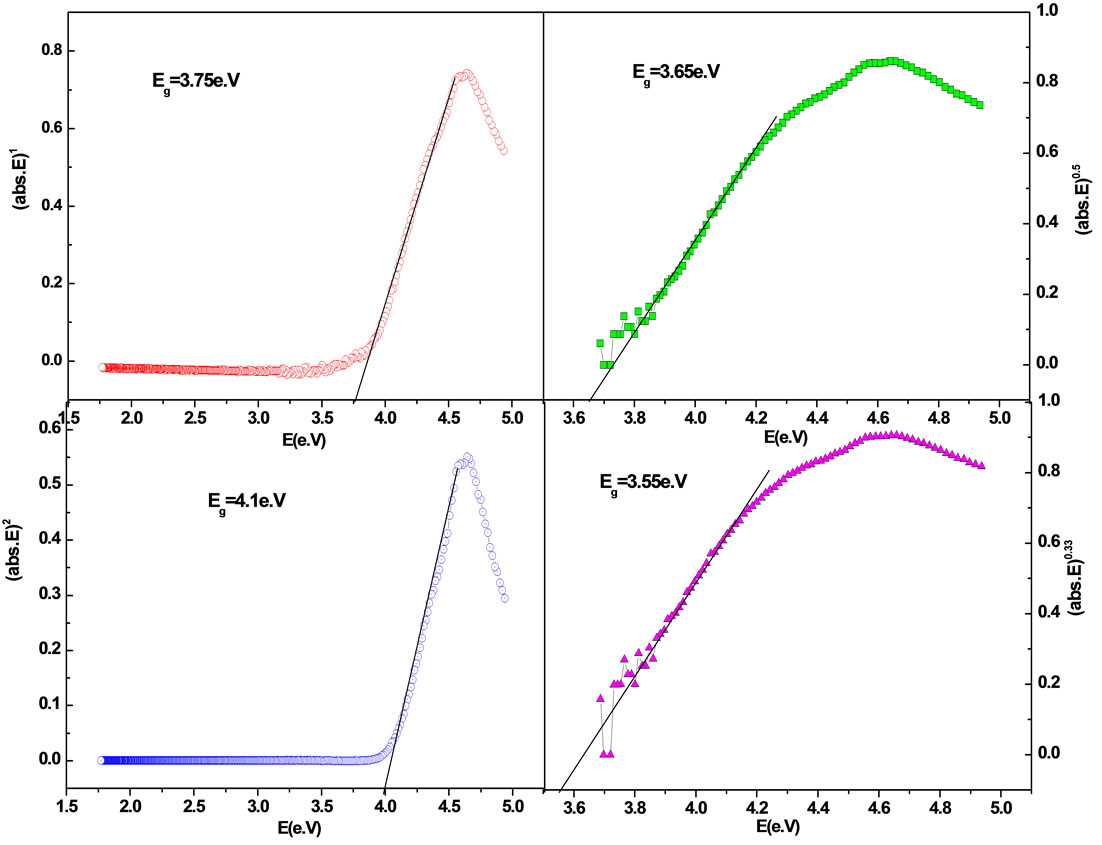
Figure 5. Shows Tauc plot for band gap calculation using different “n” values of oxypeucedanin hydrate monoacetate micro-crystal.
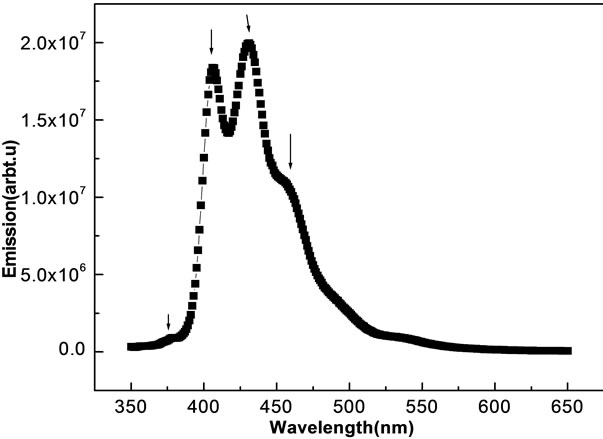
Figure 6. PL spectra of oxypeucedanin hydrate mono acetate micro-crystal after excited by 370 nm light source.
3.3. Thermal Properties
The TG-DTA curves of grown crystals are shown in Figure 7. The DTA curve shows that oxypeucedanin hydrate monoacetate micro-crystal melts at 138˚C.
The TGA curve shows a weight loss of about 18% at 137˚C due to step-wise decomposition of the compound and release of volatile substances, like ammonia and carbon dioxide. The results indicate the thermal stability of oxypeucedanin hydrate monoacetate micro-crystal in the temperature range of 35˚C - 137˚C and establish its suitability to withstand high temperatures in laser experiments. The TG curve provides a quantitative measurement of mass change associated with the transition. It shows that on melting, the material decomposes and loses mass. The curve shows a gradual mass loss. The sharpness of the endothermic peaks shows good degree of crystalinity of the crystal.
4. Conclusions
Structural, optical and thermal studies were carried out on oxypeucedanin hydrate monoacetate micro-crystals obtained by acetylation of oxypeucedanin hydrate, a furanocoumarin, isolated from the roots of Prangos pabularia. From these studies, it is revealed that these crystals show triclinic structure, are thermally stable up to 138˚C, have high transparency from 350 to about 700 nm with large band gap and high dielectric constant, show PL emission at around 403 and 430 nm. These characteristic features of oxypeucedanin hydrate monoacetate crystals make them the potential candidate for non linear optical and other device applications.
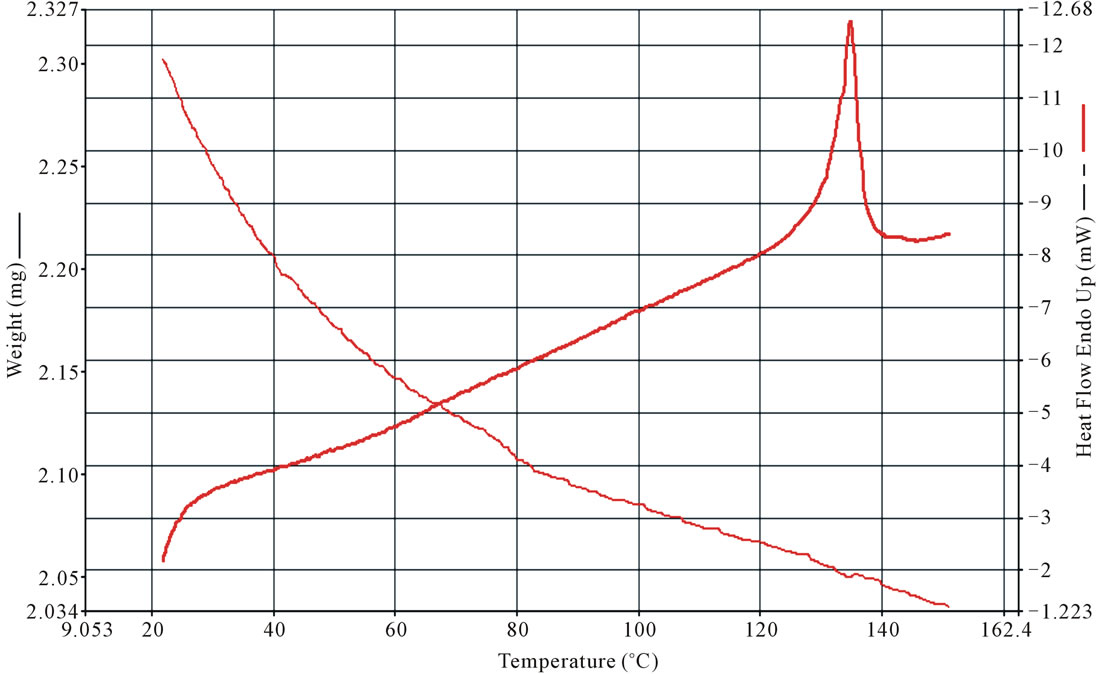
Figure 7. TGA-TA of oxypeucedanin hydrate monoacetate micro-crystal.
Therefore, the results of our studies, on oxypeucedanin hydrate monoacetate crystals clearly indicates these crystals can successfully be used in multiple applications, such as organic light emitting diodes (OLED), cladding and host material in nonlinear optical devices and organic field-effect transistors (OFET). Also, using oxypeucedanin hydrate monoacetate crystals as a gate dielectric layer, OFET devices can be fabricated, that exhibit currentvoltage characteristics with low voltages. This is a significant adventage of these crystals over other polymer or oxide based dielectrics.
5. Acknowledgements
We are very much thankful to the Director S. N. Bose National Centre for Basic Sciences Kolkata (India) for providing some experimental facilities.
REFERENCES
- Ch. Bosshard, K. Sutter, Ph. Pretre. J. Hulliger, M. Flörsheimer, P. Kaatz and P. Gunter, “Organic Nonlinear Optical Materials,” Gordon and Breach, New York, 1995.
- H. E. Huitema, G. H. Gelinck, E. Van Veenendaal, E. Cantatore, F. J. Touwslager, et al., “Aflexible QVGA Display with Organic Transistors,” IDW (Informations-Dienst-Wissenschaft), 1663.
- G. Darlinski, U. Ottger, R. Aser, H. Klauk, M. Halik, U. Zschieschang, G. Schmid and C. Dehm, “Mechanical Force Sensors Using Organic Thin-Film Transisors,” Journal of Applied Physics, Vol. 97, No. 9, 2005, Article ID 093708. doi:10.1063/1.1888046
- B. Crone, A. Dodabalapur, A. Gelperin, L. Torsi, H. E. Katz, A. J. Lovinger and Z. Bao, “Electronic Sensing of Vapors with Organic Transistors,” Applied Physical Letters, Vol. 78, No. 14, 2001, pp. 2229-2231.
- B. H. Robinson and N. C. Seaman, “The Design of a Biochip: A Self-Assembling Molecular-Scale Memory Device,” Protein Engineering, Vol. 1, No. 4, 1987, pp. 295-300.
- F.-J. M. zu Heringdorf, M. C. Reuter and R. M. Tromp, “Growth Dynamics of Pentacene Thin Films,” Nature, Vol. 412, No. 6846, 2001, pp. 517-520. doi:10.1038/35087532
- A. Turberfield, “Rapid Chiral Assembly of Rigid DNA Building Blocks for Molecular Nanofabrication,” Physics World, Vol. 43, 2003, p. 16.
- P. Damman, M. Doseire, P. Smith and J. C. Wittmann, “Orientation of p-Nitrophenol Molecules Induced by Epitaxial Crystallization on Friction-Transferred Poly (Tetrafluoroethylene) Substrates,” Journals American Chemical Society, Vol. 117, No. 3, 1995, pp. 1117-1120. doi:10.1021/ja00108a030
- P. Damman, M. Doseire, M. Bmnel and J. C. Wittmann, “Nucleation and Oriented Growth of Aromatic Crystals on Frition-Transferred Poly (Tetrafluoroe-Thylene) Layers,” Journals American Chemical Society, Vol. 119, No. 20, 1997, pp. 4633-4639. doi:10.1021/ja961173k
- G. F. Lipscomb, A. F. Garito and R. S. Narang, “An Exceptionally Large Linear Electro-Optic Effect in the Organic Solid MNA,” Journal of Chemical Physics, Vol. 75, No. 3, 1981, p. 1509. doi:10.1063/1.442157
- Y. J. Ding, X. Mu, X. Gu, Y. J. Ding, X. Mu and X. Gu, “Growth, Optical, Thermal and Dielectric Studies of an Amino Acid Organic Nonlinear Optical Material: l- Alanine,” Journal of Nonlinear Optical Physics and Materials, Vol. 9, 2000, p. 21.
- S. S. Gupte, A. Marcarno, D. Pradhan, C. F. Desai and N. Melikechi, “Laser Damage Studies in Zinc (Tris) Thiourea Sulfate: Nonlinear Optical Crystal,” Journal of Applied Physics, Vol. 91, No. 5, 2002, pp. 74-76. doi:10.1063/1.1436287
- X. Q. Wang, D. Xu, M. K. Lu, D. R. Yuan, S. X. Xu, S. Y. Guo, G. H. Zhang and J. R. Liu, “Crystal Growth and Characterization of a Novel Organometallic Nonli Near- Optical Crystal: MnHg(SCN)4(C2H6OS)2,” Journal of Crystal Growth, Vol. 224, No. 3-4, 2001, pp. 284-293. doi:10.1016/S0022-0248(01)01012-0
- M. H. Jiang and Q. Fang, “Organic and Semiorganic Nonlinear Optical Materials,” Advanced Materials, Vol. 11, No. 13, 1999, p. 1147. doi:10.1002/(SICI)1521-4095(199909)11:13<1147::AID-ADMA1147>3.0.CO;2-H
- J. Ramajothi and S. Dhanuskodi, “Crystal Growth, Thermal and Optical Studies on Phase Matchable New Organic Nlo Material for Blue-Green Laser Generation,” Journal of Crystal Growth, Vol. 289, No. 1, 2006, pp. 217-223. doi:10.1016/j.jcrysgro.2005.10.103
- N. J. Long, “Organometallic Compounds for Nonlinear Optics—The Search Fenlightenment,” Angewante Chemie, Vol. 34, No. 1, 1995, pp. 21-38. doi:10.1002/anie.199500211
- H. O. Marcy, L. F. Warren, M. S. Web, C. A. Ebbers, S. P. Velsko, G. C. Kennedy and G. C. Catella, “Second Harmonic Generation in Zinztris(thiouria) Sulphate,” Applied Optics, Vol. 31, No. 24, 1992, pp. 5051-5060. doi:10.1364/AO.31.005051
- S. Koul, “Studies on Chemical Constituents of Adhatoda vasica, Prangos pabularia and Gloriosa superbe and Chemical Transformation of Their Major Constituents,” Ph.D. Thesis, University of Jammu, Jammu, 1981.
- M. Yousaf, “Isolation and Structural Studies on the Chemical Constituents of Medicinal Plants Skimmia laureola and Witha niacoagulance,” Ph.D. Thesis, Karachi University, Karachi, 1998.
- H. Hussain, I. Ahmad, B. Schulz, S. Draeger, U. Florke, G. Pescitelli and K. Krohn, “Solid-State Circular Dichroism and Hydrogen Bonding: Absolute Configuration of Assarigenin A from Microsphaeropsis sp.,” Chirality, Vol. 23, No. 8, 2011, pp. 617-623. doi:10.1002/chir.20985
- F. A. Mir, “Growth, Structural, Optical and Electrical Study of Na-Substituted Potassium Hydrogen Tartarate Crystals,” The European Physical Journal of Applied Physics, Vol. 57, No. 2, 2012, Article ID 20202. doi:10.1051/epjap/2011110001
- N. F. Mott and E. A. Davis, “Electronic Processes in Non-Crystalline Materials,” 2nd Edition, Clarendon Press, Oxford, 1979.
- F. Moser and F. Urbach “Optical Absorption of Pure Silver Halides,” Physical Review, Vol. 102, No. 6, 1956, pp. 1519-1523. doi:10.1103/PhysRev.102.1519
NOTES
*Corresponding author.

