Paper Menu >>
Journal Menu >>
 Advances in Bioscience and Biotechnology, 2011, 2, 8-12 ABB doi:10.4236/abb.2011.21002 Published Online February 2011 (http://www.SciRP.org/journal/abb/). Published Online February 2011 in SciRes. http://www.scirp.org/journal/ABB Ca-alginate spheres behavior in presence of some solvents and water-solvent mixtures Luis G. To rr es, A ngelica Ve lasquez , Marco A . Brito-Arias Unidad Profesional Interdisciplinaria de Biotecnología. Instituto Politecnico Nacional. Av. Acueduct o s/n. Colonia Barrio la La guna Ticoman; delegacion Gustavo A. Madero 07340 D. F., Mexico. Email: LTorresB@ipn.mx Received 25 June 2010; revised 5 July 2010; accepted 12 July 2011. ABSTRACT Immobilization systems more frequently used are calcium alginate spheres. These biocatalysts have many potential applications in the immobilization of enzymes, prokaryotic cells, vegetal and animal cells, algae, organelles and mixtures of these living compo- nents. Other applications of immobilized cells imply the use of non aqueous systems. Some bioconversions are carried out in the presence of solvents such as hexane acetone or acetonitrile, or mixtures wa- ter-solvents. The aim of this work was to investigate the behaviour of Ca-alginate spheres when put in contact with different solvents (water, diesel, ethanol, methanol, acetone, n-hexane, isopropyl alcohol, THF, acetonitrile, and toluene), or solvent-water mixtures (i.e., ethanol-water), regarding the resistance of the alginate spheres after days of contact. Calcium algi- nate particles suffered different damages, depending on the solvent t h ey were put in contact. Water did not damaged the Ca-alginate structure with or without Ca present. On the other hand different solvents lost a proportion of volume, i.e., n-hexane (16%), metha- nol (19%), ethanol (19.5%), toluene (22%), diesel (34%), acetone (765), isopropyl alcohol (80%), THF and acetonitrile (total loss, total destruction). Nor the dielectric constant nor the polarity indexes were ca- pable of explaining the difference on the volume loss or total sphere destruction, except for water-ethanol mixtures. Keywords: Dielectric Constant; Ca-Alginate; Immobilization, Solvents; Spheres; Polarity Index 1. INTRODUCTION Nowadays, there is an increasing interest in producing high amounts of ethanol as an alternative fuel to fossil fuels in the whole world. In the USA, the production demand for 2012 is 7.5 billion gallons per year [1]. Ethanol is a valuable alternative to petroleum-based transportation fuels. The traditional ethanol manufac- ture was using sugar and yeast, though there are many other alternatives such as using wheat, barley, sorghum, beets, cheese whey, potatoes, and many other feed- stocks [2]. Over 90% of the ethanol produced in USA is made of corn. In opposite, in Brazil—the world’s larg- est producer—most ethanol is made from sugar cane. Other sugar sources for ethanol production are cellu- losic feedstocks, i.e. agricultural waste, forestry resi- dues and even solid municipal waste [2]. Many microorganisms can convert sugars to ethanol, and they can do it as free cells or into immobilized systems. These systems have proved to enhance pro- duction rates based in simple facts as the following: 1) immobilized systems can achieve high cell loads and maintain them for longer periods, 2) immobilization systems have a protector effect over cells, in particular when products or substrates are toxic to cells [3], and 3) these systems are flexible and allow the co-immobi- lization of different microorganisms or microorganisms and enzymes, or even cofactors. Immobilization systems more frequently used are calcium alginate spheres [4]. These biocatalysts have many potential applications in the immobilization of enzymes, prokaryotic cells, vegetal and animal cells, algae, organelles and mixtures of these living compo- nents. See Chevalier and de la Noue [5] experiments with co-immobilization of algae and bacteria. Other applications of immobilized cells imply the use of non aqueous systems. Some bioconversions are carried out in the presence of solvents such as hexane acetone or acetonitrile, or water-solvents mixtures. In recent years, it has been reported the use of im- mobilized microorganisms in order to modify the con- tent of certain molecules present in crudes and fractions, such as diesel or gasolines. Target molecules are sul- phur or nitrogen compounds, with linear, branched, cyclic or even aromatic structures.  L. G. Torres et al. / Advance s i n B io s ci e nce and Biotechnol o gy 2 (2011) 8-12 Copyright © 2011 SciRes. ABB 9 Many other systems have been reported, where Ca- alginate spheres deals with presence of non aqueous phases. Hedstrom et al. [6] used this kind of gels to immobilize Candida antartica lipase in the esterifica- tion of 2-octanol and hexanoic acid in hexane. To mention other, Hertzberg et al. [7] worked with immobilized lipase to catalyze alkyl butanoate forma- tion, transesterification reactions and hydrolysis of bu- tyl butanoate. For these reactions, the authors assessed the stability of calcium alginate beads in ethanol, ace- tone, pyridine, 2-butanone, hexane, and iso-hexane. They measured the relative volume change when beads were transferred from CaCl2 to different solvents. When calcium alginate spheres are putt in contact with different solvents and solvents-water mixtures, different behaviours are depicted. It has been reported that Ca-alginate spheres as any hydrogel are constituted mainly by water (typically 96-99%), tend to be dehy- drated in contact with alcohols, such as ethanol or iso- propyl alcohol. Other solvents cause other damages in the Ca-alginate spheres such as shrinking, deformation, dryness, and even destruction. The aim of this work was to investigate the behav- iour of Ca-alginate spheres when put in contact with different solvents (water, diesel, ethanol, methanol, acetone, n-hexane, isopropyl alcohol, THF, acetonitrile, and toluene), or solvent-water mixtures (i.e. ethanol- water), regarding the resistance of the alginate spheres after days of contact. 2. MATERIALS AND METHODS 2.1. Ca-Alginate Spheres Preparation. Ca-alginate spheres were prepared in accord to the pro- cedure previously reported by Torres et al. [8]. Na-algi- nate solutions were dropped in 0.1 M CaCl2 solutions using system in order to control the spheres diameter in about 3 mm. Spheres were prepared using calcium algi- nate solutions cont ai ning 3% of sodium alginate. 2.2. Solvents Double-distilled water was p repared in our laboratory by ultra filtration. Ethanol, methanol acetonitrile, THF, n- hexane, acetone, toluene and isopropylic alcohol were purchased either with J.T. Baker or Aldrich Chemicals. Diesel was a commercial sample, purchased in a Mexico City gas station. Some properties of those solvents such as dielectric constant and polarity index are shown at Table 1. 2.3. Average Sphere Diameters Average diameters were determined measuring 20 spheres with a veneer. Standard deviations were calcu- Table 1. Some solvent physichochemical properties. Solvent Dielectric constant DC Polarity Index* PI Water 80.0 9.0 Ethanol 24.6 5.2 Methanol 32.7 5.1 Acetonitrile 37.5 5.8 THF 4.0 4.0 n-Hexane 1.9 0.0 Acetone 21 5.1 Isopropyl alcohol20.3 3.9 Toluene 2.3 2.4 *From http: //www.chemical-ecology.net/java/solvents.htm lated using Excel program (Microsoft). 2.4. Spheres Behaviour in Presence of Different Solvents and Mixtures 200 prepared alginate beads were put in a 250 ml Er- lenmeyer flask together with 100 ml the selected solvent or mixture. Flasks were gently shaked in an orbital agi- tator for 4 days. A sample of 20 spheres was em ployed to measure initial and final averag e diameters. 3. RESULTS AND DISCUSSION Table 2 shows the changes suffered by 3% calcium alginate spheres after 3 days submerged in the different solvents. In the case of water, a 0.1 MCaCl2 solution was used to prevent Ca diffusion from the spheres to the wa- ter. Initial batches of spheres were 3.13 mm in diameter. Ca-alginate spheres were submerged in the solvents and observed after 24, 48, 72 and 96 hours. All experi- ments were carried out at room temperature (about 20 ± 2o C). Spheres submerged in water (added with CaCl2) did not show any change during the four days, as ex- pected. At the end of the process the spheres looked just like at the beginning of the experiment and the average sphere diameter decreased up to 3.047 ± 0.026 mm, giv- ing a volume loss of about 2.9%, which is negligible. That was not the case of the spheres submerged in n-hexane, where the particles were adhered to the bot- tom of the flask from the beginning, they turned yellow and at day four, all spheres seem very soft. After meas- uring the averaged diameter of 20 particles, the final diameter was of 2.904 ± 0.177 mm, resulting in a vol- ume loss of 15.9%, which is an important figure. When immersing the 200 Ca-alginate spheres on methanol, no changes were observed during the first 24 hours, but at the second day, some turbidity appeared in the flask. At third day turbidity was a little higher but no changes were observed at day 4. Final average diameter  L. G. Torres et al. / Advance s i n B io s ci e nce and Biotechnol o gy 2 (2011) 8-12 Copyright © 2011 SciRes. ABB 10 Table 2. Changes in the Ca-alginate spheres when submerged in different solvents. Elapsed time (days) Solvents 0 1 2 3 4 Final diameter Ave ± std dev Volume loss (%) CaCl2 in water NCh NCh NCh NCh Some tu rbidity 3.047 ± 0.026 2.9 n-Hexane Spheres stiked to bottom Yellowi sh sp heresYellow More yellowSoft spheres 2.904 +/- 0.177 15.9 Methanol NCh Some turbidity More turbidityNCh NCh 2.870 ± 0.032 18.8 Ethanol NCh NCh NCh NCh NCh 2.863 ± 0.072 19.5 Toluene Spheres st icke d t o the bottom NCh Some turbidity, spheres ag- glomerated NCh Very brilliant spheres 2.837 ± 0.212 21.6 Diesel Spheres sticked to bottom Some turbidity, spheres agglomer- ated NCh NCh Very brilliant spheres 2.676 ± 0.221 34.3 Acetone NCh Yellowish NCh NCh Yellow 2.003 ± 0.154 75.6 Isopropyl alcohol NCh Diameter diminu- tion NCh NCh Stiff spheres 1.788 ± 0.158 80.4 THF Diameter diminu- tion Some turbidity NCh NCh More turbidity - - Acetonitrile Spheres destruc- tion Lentil form Diameter di- minution NCh Yellow lentils - - NCh, No changes observed was 2.80 ± 0.032 mm, with a volume loss of 18.8%. The behaviour of Ca-alginate spheres in ethanol was very similar to that observed in methanol, as expected. In this case, the final sphere diameter was in average 2.863 ± 0.072 mm, and t he vol ume loss was of 19.5 mm. When Ca-alginate particles were submerged in tolu- ene, it was observed that particles were adhered to the bottom from the first day and at the end of the process, spheres were very brilliant, with an average final d iame- ter of 2.837 ± 0.212 mm and a volume loss of 21.6%. In the case of diesel, it was observed that spheres tend to agglomerate, and at the end of the process the parti- cles were very brilliant and the volume loss was of 34%. When Ca-alginate spheres were submerged for 4 days in acetone, the media turned very yellow and the final average diameter was of 2.003 ± 0.54 mm, giving a volume loss of 75.6%. When spheres were put in contact with iso-propylic alcohol, an important diameter diminu- tion was observed at the second day, and at the end of the process very stiff, small particles were produced. Final average diameter w as of 1.788 ± 0.158 mm and the volume loss was of 80.4%. The last two experiments were similar in results. When immersing the Ca-alginate spheres on acetonitrile and THF, destruction of the spheres was observed, tur- bidity appeared from the beginning of the process and it was impossible to determine for both cases the final spheres average diameter. It was considered that the sphere were unre co vered. It is well known that Ca-alginate spheres are produced by the interaction of alginic acid, which is a linear poly- saccharide and the divalent calcium, forming a structure known as the egg-box model. The destruction of the Ca-alginate gel should be promoted by the Ca loss, or either by the solubilisatio n of water in the add ed solvent. A way to analyze the effect of the characteristics of the added solvent is the measure of the polarity index and the dielectric constant of solvents. The first is the relative measure of the degree of in- teraction of the solven t with various polar test solutes. In that way, pentane and 1,1,2-trichlorotrifluoroethane have a polarity index of 0 (no interaction). Cyclopentane, heptane, hexane, iso-octane and petroleum ether have very low polarity indexes (0.1). On the other hand, dimethyl acetamide (6.5), n-methylpyrrolidone (6.7) and dimethyl sulfoxide (7.2) have high polarity indexes. In the case of water, a value of 9-10 has been reported. In the case of diesel, it is not possible to give a figure for dielectric constant. Since this is a mixture of many compounds, and diesel has a huge variability depending of the source (crude) and process employed to produce it. The dielectric constant values are referred to the measure of extent to which concentrates electrostatic lines of flux. It is the ratio of the amount of stored elec- trical energy when a voltage is applied, relative to the permittivity of a vacuum. The correct name for this pa- rameter should be relative static permittivity. With these facts in mind, the loss of volume (%) measured in every experiments were plotted as a func- tion of the polarity index in Figure 1. As noted, four of the points are around 15-20% volume loss, for polarity indexes between 0 and 5.2. For one of the points (that corresponding to water added with calcium), volume loss is negligible (2.9%). Ther e are two points with high volume losses (acetone and isopropyl alcohol, with po- larity indexes between 5.1 and 3. That means that polar- ity index do not explain why some Ca-alginate spheres shrink in some solvents more than in others. In a new intent to explain the Ca-alginate volume loss 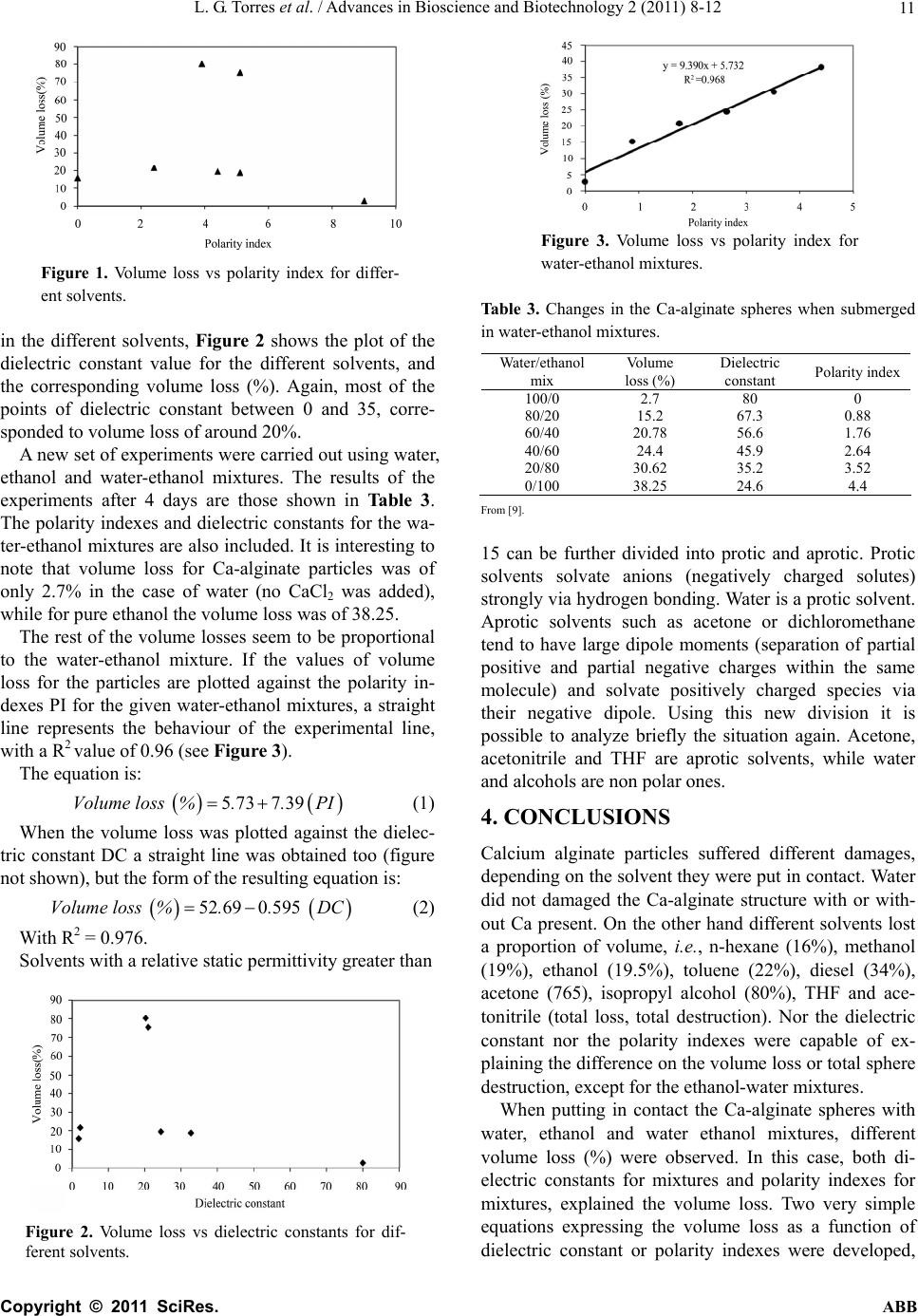 L. G. Torres et al. / Advance s i n B io s ci e nce and Biotechnol o gy 2 (2011) 8-12 Copyright © 2011 SciRes. ABB 11 Figure 1. Volume loss vs polarity index for differ- ent solvents. in the different solvents, Figure 2 shows the plot of the dielectric constant value for the different solvents, and the corresponding volume loss (%). Again, most of the points of dielectric constant between 0 and 35, corre- sponded to volume loss of around 20%. A new set of experiments were carried out using water, ethanol and water-ethanol mixtures. The results of the experiments after 4 days are those shown in Table 3. The polarity indexes and dielectric constants for the wa- ter-ethanol mixtures are also included. It is interesting to note that volume loss for Ca-alginate particles was of only 2.7% in the case of water (no CaCl2 was added), while for pure ethanol the volume loss was of 38.25. The rest of the volume losses seem to be proportional to the water-ethanol mixture. If the values of volume loss for the particles are plotted against the polarity in- dexes PI for the given water-ethanol mixtures, a straight line represents the behaviour of the experimental line, with a R2 value of 0.96 (see Figure 3). The equation is: 573739 Volume loss %..PI (1) When the volume loss was plotted against the dielec- tric constant DC a straight line was obtained too (figure not shown), but the form of the resulting equation is: 52 690 595Volume loss %.. DC (2) With R2 = 0.976. Solvents with a relative static permittivity greater than Figure 2. Volume loss vs dielectric constants for dif- ferent solvents. Figure 3. Volume loss vs polarity index for water-ethanol mixtures. Table 3. Changes in the Ca-alginate spheres when submerged in water-ethanol mixtures. Water/ethanol mix Volume loss (%) Dielectric constant Polarity index 100/0 2.7 80 0 80/20 15.2 67.3 0.88 60/40 20.78 56.6 1.76 40/60 24.4 45.9 2.64 20/80 30.62 35.2 3.52 0/100 38.25 24.6 4.4 From [9]. 15 can be further divided into protic and aprotic. Protic solvents solvate anions (negatively charged solutes) strongly via hydrogen bonding. Water is a protic solvent. Aprotic solvents such as acetone or dichloromethane tend to have large dipole moments (separation of partial positive and partial negative charges within the same molecule) and solvate positively charged species via their negative dipole. Using this new division it is possible to analyze briefly the situation again. Acetone, acetonitrile and THF are aprotic solvents, while water and alcohols are non po lar ones. 4. CONCLUSIONS Calcium alginate particles suffered different damages, depending on the solvent they were put in contact. Water did not damaged the Ca-alginate structure with or with- out Ca present. On the other hand different solvents lost a proportion of volume, i.e., n-hexane (16%), methanol (19%), ethanol (19.5%), toluene (22%), diesel (34%), acetone (765), isopropyl alcohol (80%), THF and ace- tonitrile (total loss, total destruction). Nor the dielectric constant nor the polarity indexes were capable of ex- plaining the difference on the volume loss or total sphere destruction, except for the ethanol-water mixtures. When putting in contact the Ca-alginate spheres with water, ethanol and water ethanol mixtures, different volume loss (%) were observed. In this case, both di- electric constants for mixtures and polarity indexes for mixtures, explained the volume loss. Two very simple equations expressing the volume loss as a function of dielectric constant or polarity indexes were developed,  L. G. Torres et al. / Advance s i n B io s ci e nce and Biotechnol o gy 2 (2011) 8-12 Copyright © 2011 SciRes. ABB 12 which result very interesting in predicting the volume loss by s for a given water-ethanol mixture. This is par- ticular important for the case of ethanol production by immobilized cells. REFERENCES [1] Mulson, S. (2007) Ethanol production booming on demand. Washington Post, January 23, 2007. [2] Morris, M. and Hill, A. (2007) Ethanol opportunities and questions. http://www.attra.ncat.org [3] Kewellow, H., Heipieper, H.-J. and Rehm, H.-J. (1989) Protection of bacteria against toxicity of phenol by immobilization in calcium alginate, Applied Microbio- logy and Biotechnology, 31, 283-389. [4] Smisrod, O. and Skajak-Braek, G. (1990) Alginate as immobilization matrix for cells, Trends in Biotechnology, 8, 71-78. doi:10.1016/0167-7799(90)90139-O [5] Chevalier, P. and de la Noue, J. (1998) Behavior of algae and bacteria co-immobilized in carrageninana, in a fluidized bed, Enzyme and Microbial Technology, 10, 9- 24. [6] Hedstrom, G., Backlund, S., Erickson, F. and Karlsson, S. (1998) Lipase-catalyzed stereoselective esterifications using gelatine-based hydrogels, Colloids and Surfaces. B, Biointerfaces, 10, 379-384. doi:10.1016/S0927-7765(98)00015-0 [7] Hertzberg, S., Kvittingen, L., Anthonsen, T. and Skjak- Braek, G. (1992) Alginate as immobilization matrix and stabilizing agent in a two-pase liquid system: Application in lipase-catalysed reactions, Enzyme and Microbial Technology, 14, 42-47. doi:10.1016/0141-0229(92)90024-I [8] Torres, L.G, Sanchez-de-la-Vega, A., Beltran, N.A. and Jimenez, B.E. (1998) Production and characterization of a Ca-alginate biocatalyst for removal of phenol and chlorophenols from wastewaters, Process Biochemistry, 33, 625-634. doi:10.1016/S0032-9592(98)00026-0 [9] Farji, M., Farajtabar, A. and Oharib, F. (2009) Deter- mination of water-ethanol mixtures autotpotolysis cons- tants and solvent effect. Journal of Applied Chemistry Research, 9, 7-12. |

