Advances in Bioscience and Biotechnology
Vol. 4 No. 7 (2013) , Article ID: 34153 , 4 pages DOI:10.4236/abb.2013.47102
High frequency multiplication of shoots using axillary buds for production of elite lines of Stevia rebaudiana
![]()
Department of Biotechnology, Kristu Jayanti College, Bangalore, India
Email: *kavyashree@kristujayanti.com
Copyright © 2013 Kavyashree Rangappa, Divya Shalini Aind. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 28 February 2013; revised 28 March 2013; accepted 5 April 2013
Keywords: Stevia rebaudiana; Endemic; Axillary Bud; Clonal Multiplication
ABSTRACT
An efficient repeatable protocol has been developed for rapid clonal multiplication of an endemic, perennial herb Stevia rebaudiana using juvenile axillary buds from in vivo grown seedlings. MS basal medium supplemented with BAP (8.88 µM) and NAA (5.37 µM) was found to be the most suitable medium for initiation of shoots with 92% response. The repeated subculture resulted in high-frequency shoot multiplication (9.82 ± 1.93) at the average rate of 3-fold per subculture. The axenic multiple shoots were rooted ex-vitro on horticultural grate soilrite mix-peat:perlite:vermiculate (1:1:1 v/v) with half strength MS salts fortified with NAA (5.37 µM). The complete plantlets were successfully established in soil with 86% survival frequency. The statistical analysis of the data pertaining to multiple shoots and root formation revealed significant difference between and within the treatments.
1. INTRODUCTION
Stevia rebaudiana is a perennial herb, native to the highlands of Paraguay [1]. It belongs to the family Asteraceae and it is one of the 154 members of the genus Stevia. S. rebaudianais popularly known as sugar substitute, sweet weed or sugar leaf. It is a natural alternative source to traditional sugar (sucrose) obtained from sugarcane, sugar-beet etc. [2]. The leaves of Stevia rebaudiana are the source of the diterpene glycosides, stevioside and rebaudioside, which are reported to be 100 - 300 times sweeter than sucrose [3,4]. It is one of the important herb for the mankind in response to today’s major health concerns like diabetes, hypertension, etc.
The cultivation of Stevia on commercial basis is done by seed, stem cutting or division of mother plants in green house during winter. The poor seed germination rate and lower success of vegetative propagation by stem cuttings coupled with requirement of enough stocks of stem cuttings and higher labor inputs are the major limiting factors in its large scale cultivation [5-7]. A suitable alternative method to obtain sufficient number of plants within short time duration is the use of in vitro cultures. Further, organ differentiation in plants is regulated by an interplay of auxins and cytokinins [8]. A higher cytokinin-to-auxin ratio promotes shoot formation and the synergistic effect of cytokinin and auxin ratio in the medium enhances the rate of multiplication [9]. The micropropagation of plants through axillary bud culture allows recovery of genetically stable, elite true-to-type progeny [10,11]. Stevia can form multiple shoots from nodal explants, which are convenient type of culture for multiplication on large scale. There are few reports of in vitro clonal propagation of Stevia using leaf, nodal, internodal segments and shoot tips [2,12-17]. Hitherto, there are no reports on high-frequency multiplication of shoots followed by ex vitro rooting of Stevia. In this direction, an attempt has been made in the present study to develop a simple and economical regeneration protocol for rapid rate of shoot multiplication followed by rooting ex vitro thereby eliminating a step of in vitro rooting.
2. MATERIAL AND METHODS
Plant Material
Stevia rebaudiana Bertoni plants were collected from the APD Horticulture Training Centre, Kothanur, Bangalore and planted in the garden of Kristu Jayanti College, Bangalore. The juvenile axillary bud explants were collected from the 2 months old mature in vivo grown seedlings.
3. EXPERIMENTAL PROCEDURE
3.1. Surface Sterilization
The axillary bud explants were surface sterilized by washing thoroughly under running tap water for 10 min and then immersed in 0.05% bavistine for 1 hour. Excess of bavistine was washed thoroughly with distilled water. The surface sterilized explants thus obtained were rinsed in 70% alcohol for 30 seconds in LAF chamber, disinfected in 0.01% mercuric chloride (HgCl2) for 1 minute and rinsed several times in sterile water to remove the traces of HgCl2.
3.2. Culture Media and Growth Conditions
The sterilized axillary were cut into appropriate sizes (0.8 - 1.0 cm) and were cultured on Murashige and Skoog’s basal medium (MSBM) supplemented with different concentrations of BAP and NAA ranging from (4.44 - 17.76 µM) and (2.68 - 10.74 µM) respectively to study their response on initiation and multiplication of axillary buds (Table 1). The cultures were maintained in culture room at 25˚C ± 2˚C under 16/8 (light/dark cycle) photoperiod and irradiance of 1000 lux provided by cool white florescent lamps. The axillary buds from the proliferated shoots were sub-cultured repeatedly to obtain large number of multiple shoots.
The well-developed multiple shoots were transferred to culture bottles containing horticultural grade soilrite mix-peat:perlite:vermiculate (1:1:1 v/v) with half strength MS salts supplemented with different concentrations of auxin a-Naphthalene Acetic Acid (NAA), Indole Acetic Acid (IAA) and Indole Butyric Acid (IBA) ranging from 2.68 - 10.74 µM; 2.85 - 11.40 µM and 2.46 - 9.84 µM respectively (Table 2) to study their effect on ex vitro rooting. The rooted multiple shoots were taken out from bottle and transferred to pots containing tissue culture grade soilrite mix-peat:perlite (3:1 v/v) without any adjuvants for profuse rooting and simultaneous acclimatization. The acclimatized complete plantlets were transferred to soil and their survival frequency was calculated.
4. STATISTICAL ANALYSIS
The experimental data of the present investigation was analyzed statistically by one-way analysis of variance (ANOVA) to determine the variation in the number of multiple shoots and roots between and within the treatments (Tables 1 and 2). For each treatment 10 replicates were used and each experiment was repeated thrice.
5. RESULT AND DISCUSSION
In the present study, in vitro regeneration of shoots from the axillary buds of Stevia using different concentration

Table 1. Effect of different concentrations of growth regulators on initiation and multiplication of shoots from axillary bud explants of Stevia rebaudiana.
and combination of BAP and NAA varied markedly. BAP, a synthetic cytokinin, was tried as it the most useful, reliable and cheapest cytokinin and should be tested first for a new system [8]. Further, NAA was tried of the various auxins available as IAA is least stable in the medium and 2,4-D is avoided because of its strong tendency to induce callusing. The shoot initiation from the axillary bud was observed within 2 - 3 days of culture, which developed 4 - 5 leaves after 10 days (Figure 1(a)) in all the concentrations of growth regulators tried with varied percentage of response (42% - 92%). The highest percentage of response was observed on MSBM fortified with BAP (8.88 µM) and NAA (5.37 µM) whereas least percentage of response was noticed on media supplemented with BAP alone and also on MSBM with BAP (4.44 µM) and NAA (8.05 µM) (Table 1). The initiated shoots elongated within 20 - 30 days of culture (Figures

Table 2. Effect of different auxins on ex vitro rooting of in vitro raised plants derived from axillary bud of Stevia rebaudiana.
1(b) and (c)) which on repeated subculture produced multiple shootsafter 30 - 40 days of culture (Figures 1(d) and (e)). The statistical analysis of the data revealed that there exist significant differences between and within the treatment with respect to the number of shoots per explants. The highest number of multiple shoots (9.82 ± 1.93) was noticed on the same medium with highest percentage of response. Analysis of the results depicted that fairly good number of multiple shoots was noticed on all the medium that contained cytokinin/auxin ratio 2:1. Hence, 5.58 ± 0.71 and 4.42 ± 0.52 with 86% and 72% response was noticed on MSBM fortified with BAP (13.32 µM), NAA (8.05 µM) and BAP (17.76 µM), NAA (10.74 µM) respectively. Increased concentration of BAP resulted in retardant plants of Stevia [18] and also low concentration of cytokinin BAP with high concentration of auxin NAA showed poor responseindicating that the synergistic effect of cytokinin and auxin ratio of 2:1 is ideal for good shoot proliferation. This agrees with the findings of scientists who have reported 2:1 cytokinin/auxin ratio for shoot initiation and multiplication [8,19]. However, MSBM with BAP (13.32 µM) and NAA (5.37 µM) was an exception to the above findings as 84% response with 6.18 ± 0.74 multiple shoots was noticed in 3:1 cytokinin/auxin ratio. This enhancement in the rate of multiplication is due to the synergistic effect of cytokinin and auxin ratio in the medium. Similar observations were made by scientist who have suggested that to achieve high frequency of shoot multiplication it is necessary to supplement cytokinin and auxin to the culture medium [9]. However, the findings reported in the present study is in contrast with the findings of scientists who have reported maximum shoot regeneration from nodal segments of Stevia on MS medium supplemented with BAP alone [20,21].
Ex vitro rooting of axenic multiple shoots was achieved on horticultural grade soilrite mix-peat:perlite:vermiculate (1:1:1 v/v) containing half strength MS salts with NAA (5.37 µM) when compared to other concentrations of growth regulators tried (Figure 1(f)). The statistical analysis of the data depicted that highest number of roots per plantlet (5.92 ± 0.24) in NAA (5.37 µM) and lowest response (2.14 ± 0.22) in IAA (2.85 µM and 11.40 µM). Similar results have been observed with NAA (8.05 µM) [22], with NAA (5.37 µM) [23], with NAA (2.68 µM) [20]. However, their results are underin vitro conditions unlike our report, which is in ex vitro condition. Further, the rooted plants were acclimatized in pots using tissue culture grade soilrite mix-peat:perlite (1:1 v/v) without any adjuvants. This technique eliminates a step of in vitro rooting thereby reducing the overall cost of the micropropagated plants. Further, production of plantlets with profuse rooting ex vitro is more important for transferring regenerants to soil. The ex vitro rooted plants were established in soil with 86% survival frequency.
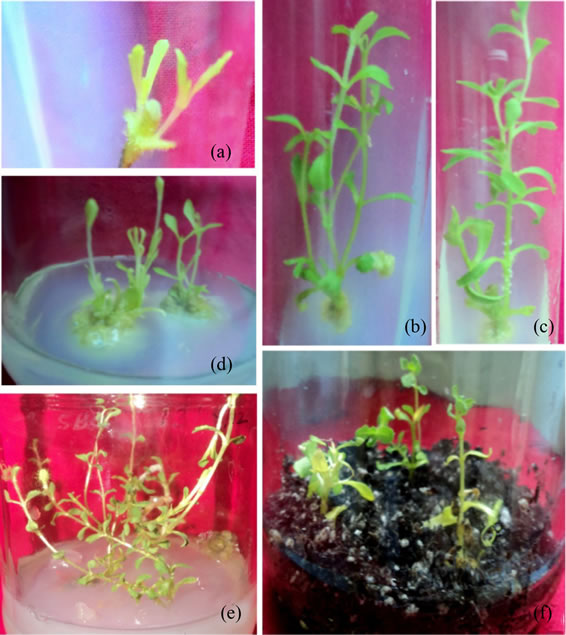
(a) Initiation of shoots; (b), (c) Elongation of shoots; (d) Formation of multiple shoots; (e) Healthy, elongated and robust multiple shoots; (f) Ex vitro rooting of multiple shoots.
Figure 1. Rapid clonal multiplication using axillary bud of Stevia rebaudiana.
In conclusion, the protocol outlined in this paper for micropropagation of Stevia is an economical, faster and promising approach for large-scale multiplication that can be utilized to obtain healthy elite, genetically identical clones of Stevia for commercial exploitation.
6. ACKNOWLEDGEMENTS
The financial assistance received by Vision Group of Science and Technology (VGST), Department of Information Technology, Biotechnology and Science and Technology, Government of Karnataka under SMYSR scheme is gratefully acknowledged.
![]()
![]()
REFERENCES
- Brandle, J. E. and Rosa, N. (1992) Heritability for yield, leaf: stem ratio and stevioside content estimated from a landrace cultivar of Stevia rebaudiana. Canadian Journal of Plant Science, 72, 1263-1266. doi:10.4141/cjps92-159
- Anbazhagan M., Kalpana, M., Rajendran, R., Natarajan, V. and Dhanavel, D. (2010) In vitro production of Stevia rebaudiana Bertoni. Emirates Journal of Food and Agriculture, 22, 216-222.
- Ishima, N. and Katayama, O. (1986) Sensory evaluation of stevioside as a sweetener. Reports of National Food Research Institute, 31, 80-85.
- Tanaka, O. (1982) Steviol-glycosides: New natural sweeteners. TrAC Trends in Analytical Chemistry, 1, 246-248. doi:10.1016/0165-9936(82)80079-4
- Carneiro, J.W.P., Muniz, A.S. and Guedes, T.A. (1997) Green house bedding plant production of Stevia rebaudiana (Bert.). Canadian Journal of Plant Science, 77, 473- 474. doi:10.4141/P96-166
- Debnath, M. (2008) Clonal propagation and antimicrobial activity of an endemic medicinal plant Stevia rebaudiana. Journal of Medicinal Plants Research, 2, 45-51.
- Taware, A.S., Harke, S.N., Mukadam, D.S., Chavan, A.M. and Taware, S.D. (2010) Effect of different extracts of callus and plantlets of Stevia rebaudiana (Bertoni) on seed germination of some agricultural crops. African Journal of Biotechnology, 9, 6675-6683.
- Bhojwani, S.S. and Razdan, M.K. (2004) Plant tissue culture: Theory and practice. Revised Edition, Elsevier Publication, Amsterdam.
- Sugimura, Y., Adachi, T., Kotani, E. and Furusawa, T. (1998) Shoot bud formation and plantlet regeneration from the basal tissues of mulberry leaves. The Journal of Sericultural Science of Japan, 67, 421-424.
- George, E.F. and Sherrington, P.D. (1984) Plant propagation by tissue culture. Exegetics Ltd., Eversley, Basingstoke, 39-71.
- Sairkar, P., Chandravanshi, M.K., Shukla, N.P., Mehrotra N.N. (2009) Mass production of an economically important medicinal plant Stevia rebaudiana using in vitro propagation techniques. Journal of Medicinal Plants Research, 3, 266-270.
- Ahmed, M.B., Salahin, M., Karim, R., Razvy, R.A., Hannan, M.M., Sultana, R., Hossain, M. and Islam, R. (2007) An efficient method for in vitro clonal propagation of a newly introduced sweetener plant (Stevia rebaudiana Bertoni.) in Bangladesh. American-Eurasian Journal of Scientific Research, 2, 121-125.
- Salim, M.U., Shaheed, M.H.C., Mouoztaba, M.M.K., Belal, M.U., Ahmed, R. and Baten, M.A. (2006) In vitro propagation of Stevia rebaudiana Bert in Bangladesh. African Journal of Biotechnology, 5, 1238-1240.
- Yukiyoshi, T., Shigeharu, N., Hiroshi, F. and Mamoru, T. (1984) Clonal propagation of Stevia rebaudiana Bertoni by stem-tip culture. Plant Cell Reports, 3, 183-185. doi:10.1007/BF00270195
- Giridhar, P., Sowmya, K.S., Ramakrishna, A. and Ravishankar, G.A. (2010) Rapid clonal propagation and stevioside profiles of Stevia rebaudiana Bertoni. International Journal of Plant Developmental Biology, 4, 47-52.
- Das, K., Dang, R. and Rajasekharan, P.E. (2006) Establishment and maintenance of callus of Stevia rebaudiana Bertoni under aseptic environment. Natural Product Radiation, 5, 373-376.
- Das, A, Gantait, S. and Mandal, N. (2011) Micropropagation of elite medicinal plant: Stevia rebaudiana Bert. International Journal of Agricultural Research, 6, 40-48.
- Sivaram, L. and Mukundan, U. (2003) In vitro culture studies on Stevia rebaudiana. In Vitro Cellular & Developmental Biology—Plant, 39, 520-523. doi:10.1079/IVP2003438
- George, E.F. (1996) Plant propagation by tissue culture part 2: In practice. 2nd Edition, Exegetics Ltd., Westbury.
- Rafiq, M., Dahoti M.U., Mangrios, S.M., Naqvi, H.A. and Qarshi, I.A. (2007) In vitro clonal propagation and biochemical analysis of field established Stevia rebaudiana Bertoni. Pakistan Journal of Botany, 39, 2467-2474.
- Thiyagarajan, M. and Venkatachalam, P. (2012) Large scale in vitro propagation of Stevia rebaudiana (bert) for commercial application: Pharmaceutically important and anti-diabetic medicinal herb. Industrial Crops and Products, 37, 111-117. doi:10.1016/j.indcrop.2011.10.037
- Hossain, M.A., Kabir, A.H.M, Jahan, T.A. and Hasan, M.N. (2008) Micropropagation of Stevia. International Journal of Sustainable Crop Production, 3, 1-9.
- Steven, M.S., Gail, B.M. and Christopher, W.W.B. (1992) Stevioside biosynthesis by callus, root, shoot and rootedshoot cultures in vitro. Plant Cell Tissue and Organ Culture, 28, 151-157.
NOTES
*Corresponding author.

