Journal of Minerals and Materials Characterization and Engineering
Vol.1 No.5(2013), Article ID:36682,5 pages DOI:10.4236/jmmce.2013.15033
Recovery of Iron Ore Tailings by Column Flotation
Centro de Desenvolvimento da Tecnologia Nuclear (CDTN), Comissão Nacional de Energia Nuclear, Belo Horizonte, Brazil
Email: plinio@cdtn.br
Copyright © 2013 Plinio Eduardo Praes et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 28, 2013; revised July 3, 2013; accepted July 30, 2013
Keywords: Flotation; Iron Ore; Slimes
ABSTRACT
The exploration of iron ore in small mining companies results in a large amount of unrecovered fine material, representing 35% of the run of mine. Millions of tons of useful minerals are discarded every year into tailings, incurring operation costs, raising production losses and environmental impact. In addition to the disposal of 300 t/h of this material, millions of tons are already stored in tailings. CDTN carried out a study of the process in a column flotation pilot plant installed in the mining facilities with a slime sample, with a view to recovering this sludge using the reverse flotation technique. The aim of this experiment was to obtain a final concentrate whose Fe content assigned 66% with a SiO2 content below 0.8% and P lower than 0.03%. The results showed that the ultrafine flotation is an economical alternative if the improvement of the recovery in the mineral sector is concerned. Ultrafine flotation can also be assessed when dealing with reduction and treatment of effluent discharged in the environment.
1. Introduction
The concentration of iron ore by flotation is a technique consolidated worldwide for mineral particles ranging from 10 to 250 μm [1].
In Brazil, all the main pellet-feed producers use reverse flotation, being the tailing made up mostly of quartz and a concentrate constituted of iron oxides and iron hydroxides. The most used reagents are amine and corn starch, which act respectively as collector and depressant [1-4]. According to Araújo and Viana [5], flotation columns can be employed in tandem with conventional cells or isolated.
Iwasaki [6] mentions three factors contributing to the success of flotation in the concentration of iron ores: it presents better performance in the concentration of low content oxidised ores; it enables the reduction of contents in silicon of pre-concentrates obtained through magnetic separation; it is the most indicated process for the production of concentrates dedicated to metallurgic processes of direct reduction. Rabelo [7] points out the strong positive impact of flotation on environmental issues, for it allows the recovery of large masses of fine fractions of ores having low contents of iron rejected in the process concerning the production of granulates and sinter-feed throughout the years.
The processes of iron ore flotation being either by means of mechanical cells or columns, are fed by particles whose sizes vary 10 and 150 µm, the ultrafine particles (slimes) are removed by means of cyclone, for they affect the process in a negative way, and the top size is limited to values between 5% and 10% above 150 µm.
A strong world demand for iron ore observed in the last years have contributed to a rise in the production capacity in the operating mines, to the implementation of new mines in the traditional production sites and to the interest in proper use of materials by then considered refuse and not used. A few mining companies in the “Quadrilatero Ferrifero Area” still operate with run of mine crushing and rating by sieving, which leads to the use of material with a grain size above 1/4”, representing approximately 30% of the run of mine. The remaining material, which represents 70% of the total mass, having a grain size below 1/4”, is separated again by means of a spiral type classifier, creating two fractions: the sinter feed, smaller than 1/4” and bigger than 0.15 mm, and the pellet feed, smaller than 0.15 mm.
In the case of this work, in the beginning the feeding in the industrial facilities was 800 t/h, being that around 35% of this material, made up of tailings of the magnetic separation, having an Fe content of around 35% and SiO2 generally above 40%, were not recovered, they were stored in tailings, resulting in a financial and environmental issue for they were a liability demanding continuous monitoring. The industrial facility is made up of stages of crushing, screening, spiral concentration and magnetic separation. It is worth highlighting that the Fe and SiO2 contents mentioned above may vary according to the origin of the ore, i.e. of the mine front operation in a certain moment.
This paper presents the results of the study of the concentration by flotation carried out with a fraction of iron ore made up of the magnetic separation tailing. The study into the recovery of this material carried out in a pilot unit scale had as its main objective, the production of an iron concentrate meeting standards required for commercialisation i.e. Fe and SiO2 content of around 66% and 1.0%, respectively and the highest possible recovery in the flotation.
2. Experimental
2.1. Materials and Methods
The material used in the flotation tests comes from the tailing of the magnetic separator of the industrial site, as the samples presented in the photographs in Figure 1. Firstly the sample went through a circuit, made up of a 9 inch spiral classifier made by Denver, and a 40.0 mm Krebs cyclone, operating at a pressure 60 psi, with an apex diameter of 2.0 mm and 13.0 mm vortex. The pulp flow was approximately 200 L/h and the solid one 50 kg/h. At the end of the process, the overflow of the cyclone was discarded and the underflow accounted for the feeding of the columns.
Table 1 and Figure 2 show the granulochemical distribution of Fe and SiO2 of the overflow and underflow of the cyclone. In accordance with the results, the ore has a fine grain size, with 66.2% of the mass and 40.1% of the Fe containing in the fractions below 400 Tyler mesh (37 µm).
The complete chemical analysis of the samples of ore studied is presented in Table 2.
2.2. Tests of Column Flotation in a Pilot Unit
In the rougher flotation, a column having 5.1 cm in diameter and a total height of 6.0 meters was used. The control of the interface was carried out by means of adjustment of the pulp flow from the fraction not floated, as shown in Figure 3. This adjustment used to be done by means of signals emerging from two pressure sensors installed in the column and processed in a CD 600 digital control set. The compressed air, before being introduced to the column, went through a filter, a pressure regulator and finally through a flow meter and a control valve. The


Figure 1. View of the tailing tanks containing the fine refuse of iron ore.
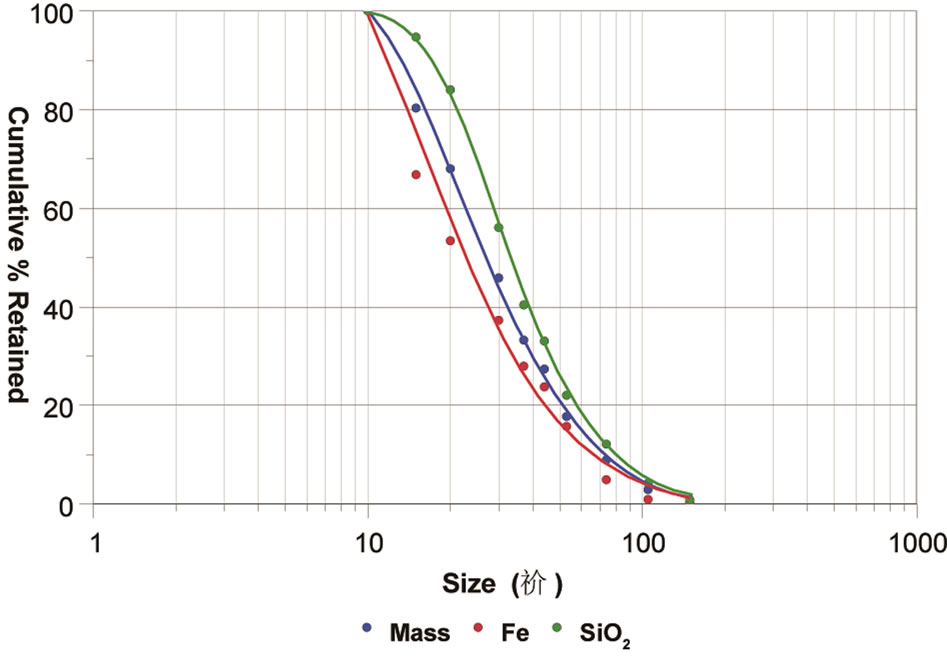
Figure 2. Granulochemical distribution of the cyclone underflow.
water used in the washing was measured and controlled by means of a volumetric flow meter and a control valve.
Based on early works using iron ore of similar characteristics in laboratory tests, the following variables were studied: type and dosage of collectors, type and dosage of depressant, pH values, feed solid percent, superficial air rate. The solid percent in the conditioning phase and in the column feeding was kept at approximately 35% and 25% respectively. The pH adjustment made with sodium
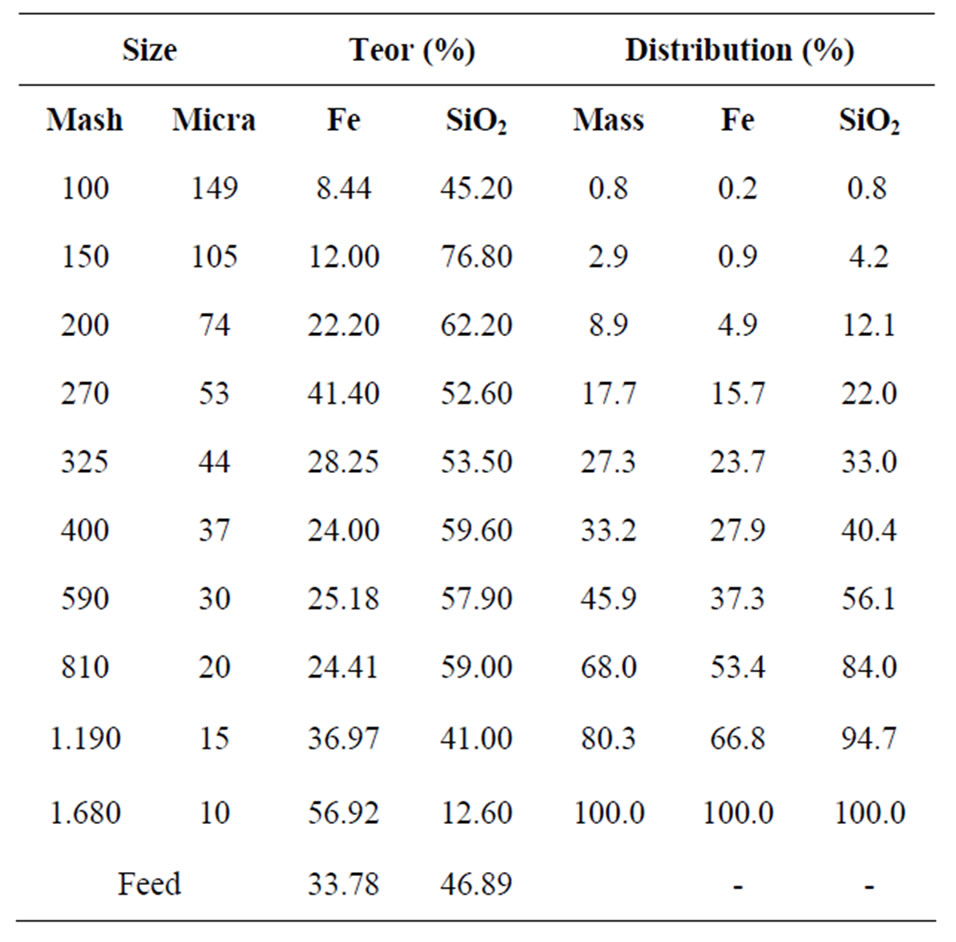
Table 1. Granulochemical distribution of the underflow of the cyclone.
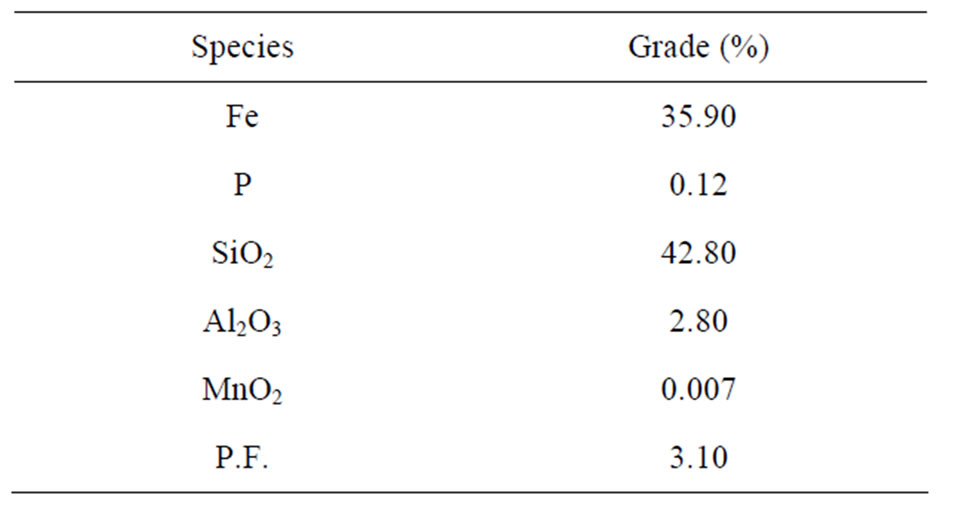
Table 2. Chemical composition of the slimes.
hydroxide.
3. Results and Discussions
Further on, the main results obtained in the study will be analyzed. The function of a collector in the flotation process is to make the surface or the minerals to be floated hydrophobic, thus separating them from the hydrophilic materials. The initial flotation tests in column were performed in order to assess the effect of six different collectors of the type primary amine and secondary amine— the results are presented in Figure 4. One can notice that on the whole, all the collectors tested presented a satisfactory performance in the flotation of the fines of iron ore of the sample, the increase of the collector dosage accounted for a increase in the content of iron and a considerable reduction in the SiO2 content in the iron concentrate, while the recovery of the mass and of Fe fell.
It is important to add a depressant agent to the iron minerals in the iron ore reverse flotation. In this work, corn starch and CMC (carboxymethylcellulose) were used. The results presented in Figure 5 showed that under the condition studied, the increase of the depressant dosage resulted in a rise in the mass and Fe recovery, as well as a reduction in the SiO2 content in the concentrate. The CMC presented selectivity in the separation of the iron minerals and silicates slightly higher than the corn starch, however at a significantly higher price.
In the flotation process, the pH is an important variable, for is alter the characteristics of the pulp by means of a variation in the concentration of the ions present. This allows the alteration and adsorption of the collector and/or depressant over the surface of the mineral particles. In this work, tests were carried out by varying the
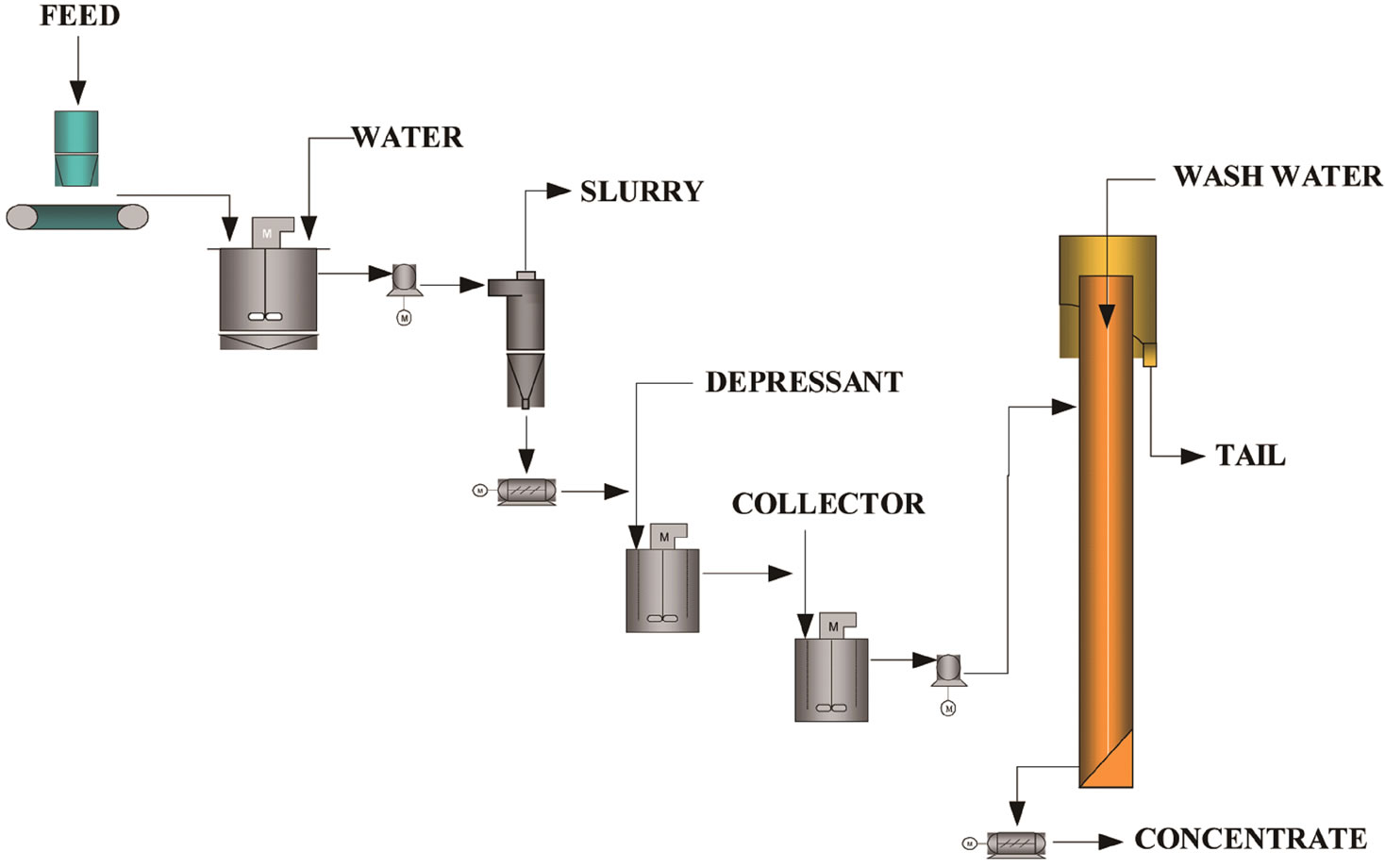
Figura 3. Flowsheet of column flotation pilot unity.
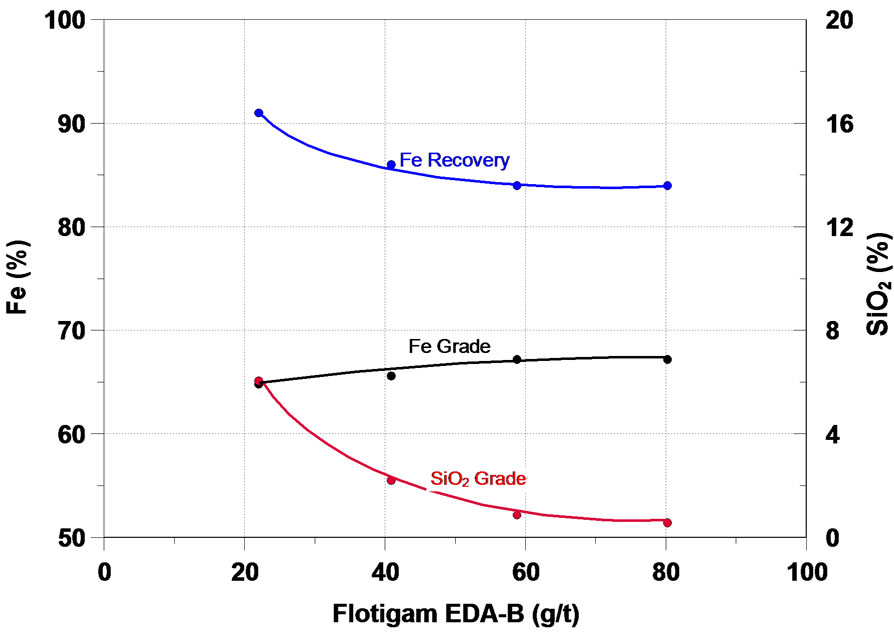
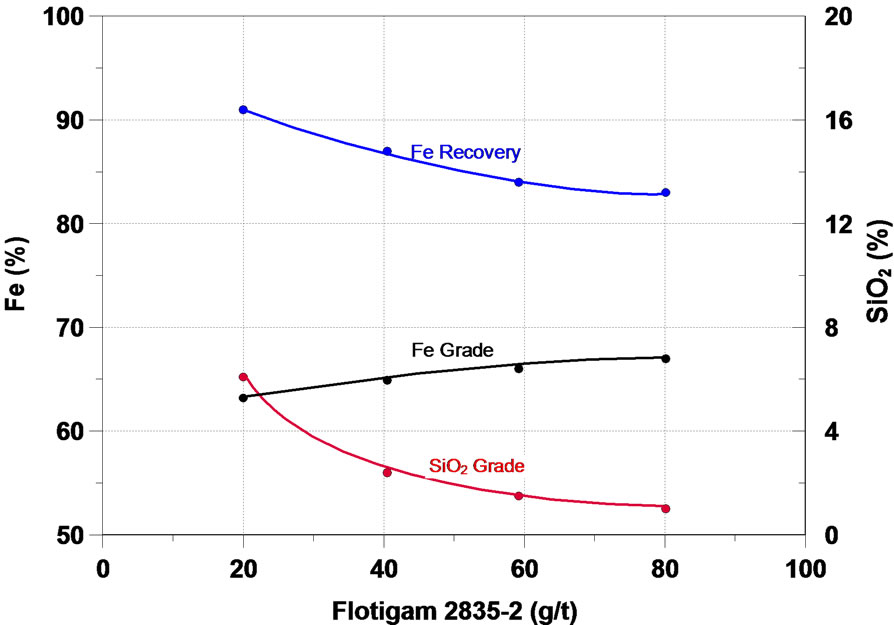
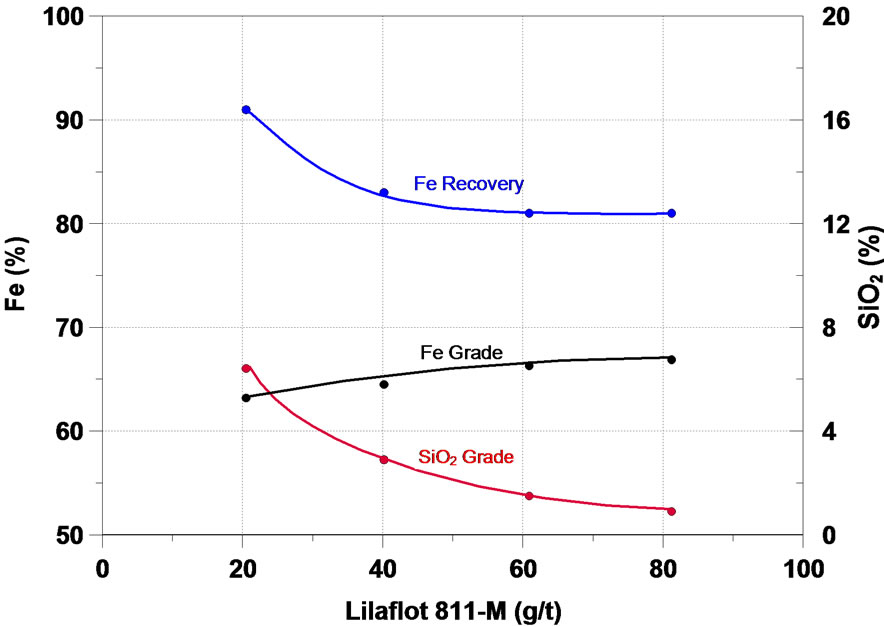
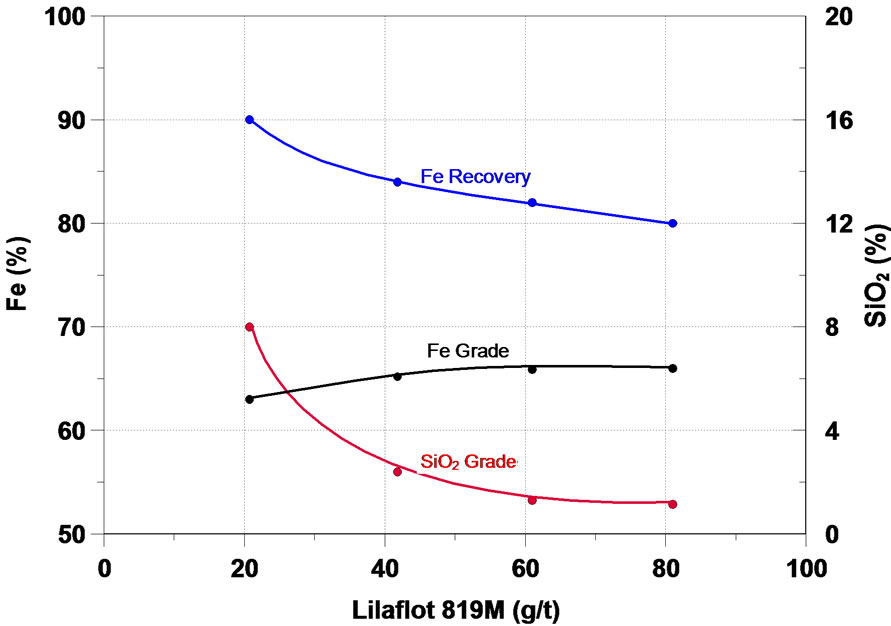
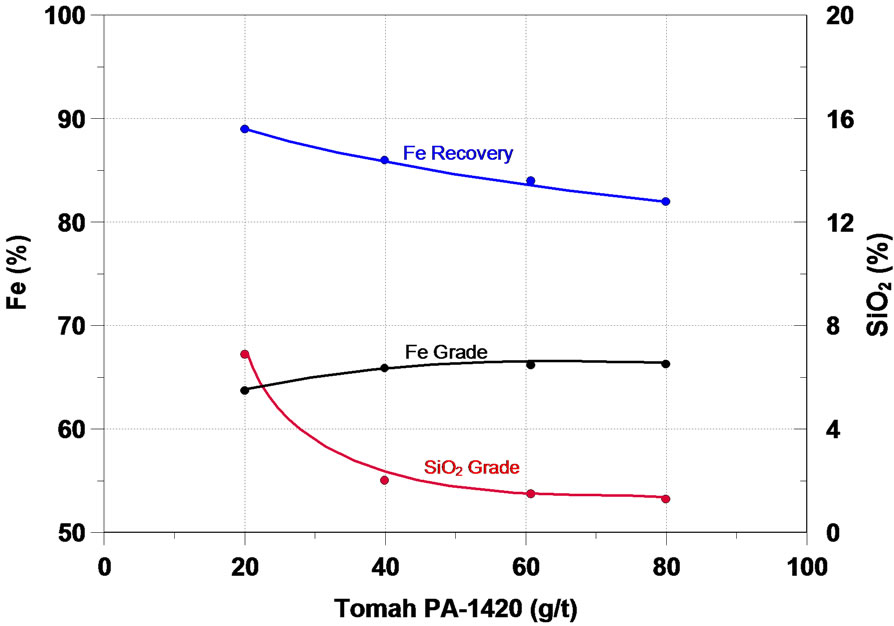
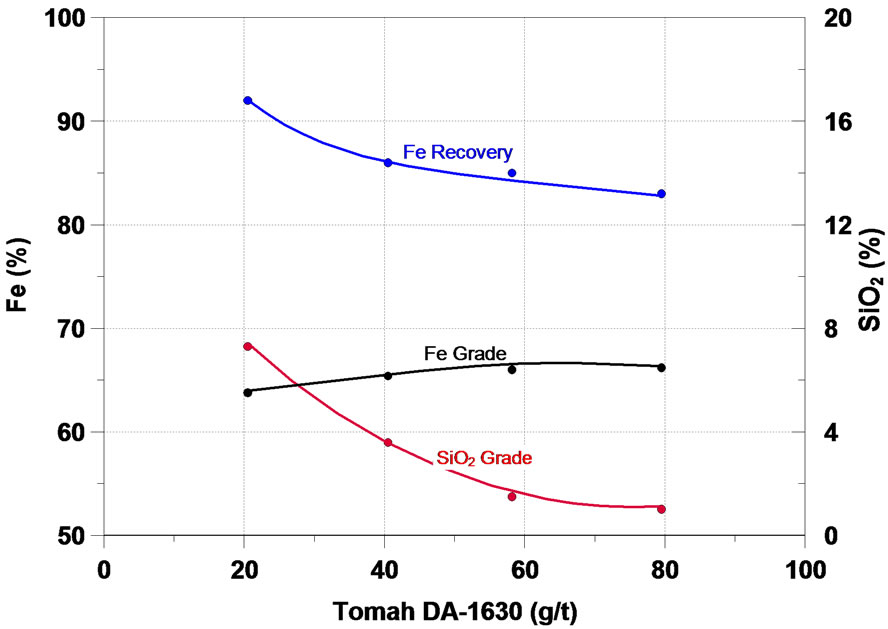
Figura 4. Effect of collectors in iron flotation.
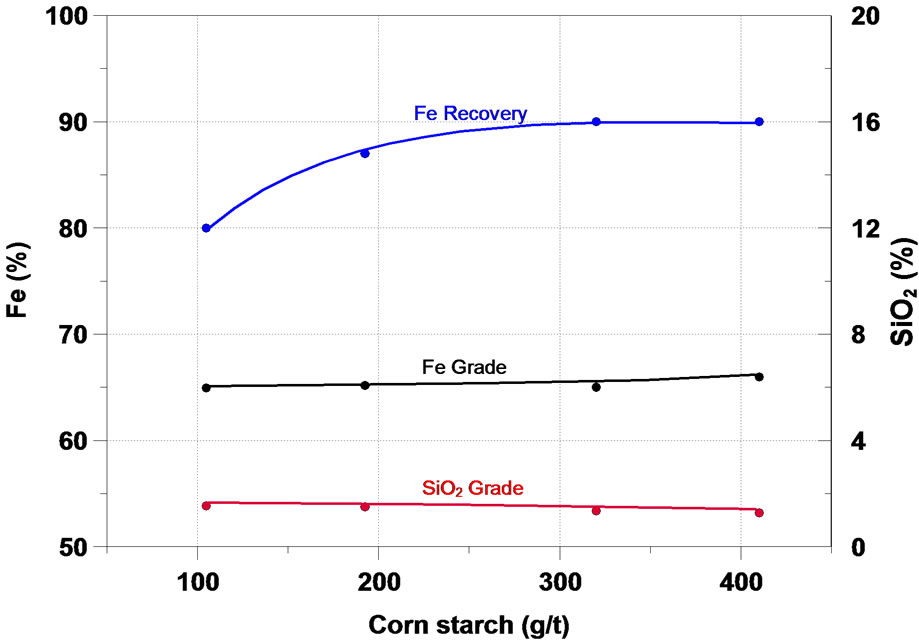
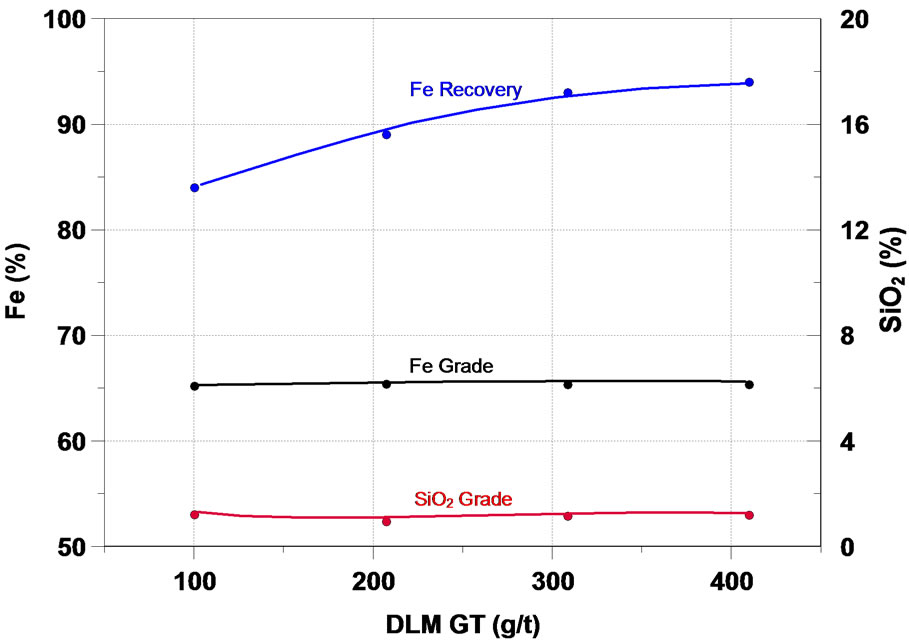
Figura 5. Effect of depressant in iron flotation.
 (a)
(a) 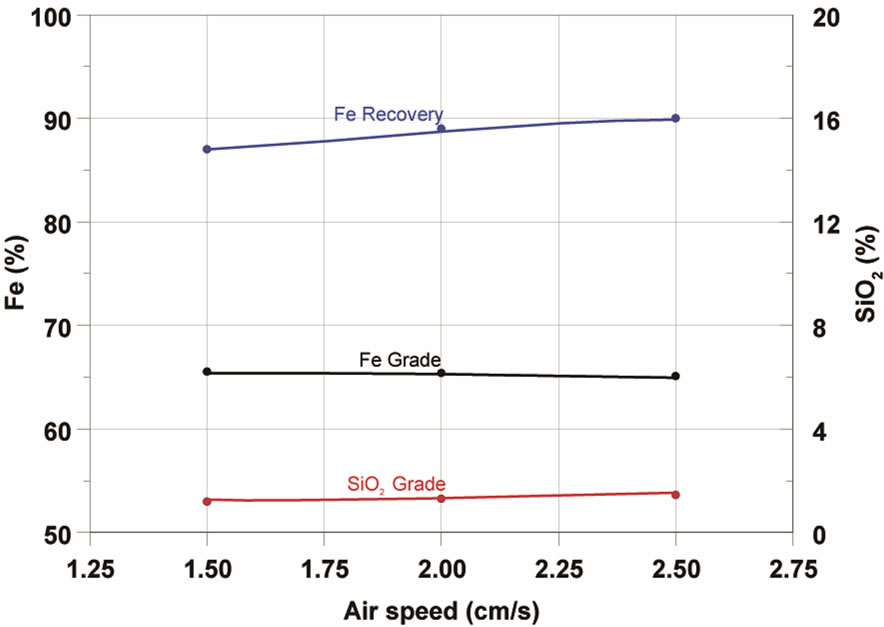 (b)
(b)
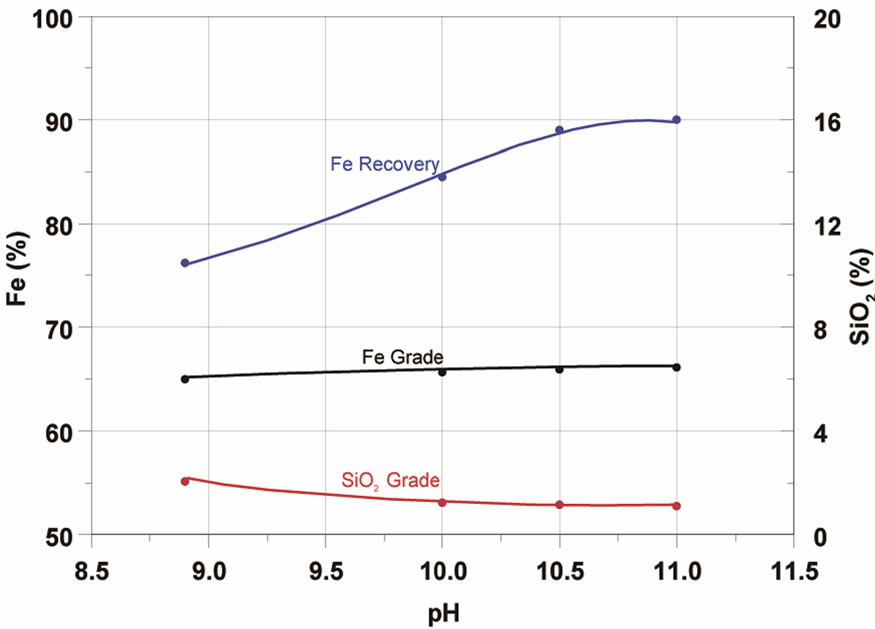
Figura 6. Effect of pH (a) and air speed (b) in iron flotation.
pH values in levels usually applied in the reverse flotation of iron ore, i.e. between 8.9 and 11.0. The results presented in Figure 6 show that the rise in pH led to a significant rise in the recovery of Fe in the concentrate. The content of SiO2 diminished for pH values up to 10.5 and the Fe content remained practically stable throughout the range studied.
The air flow usually exerts an important influence on the column floatation process, especially on the recovery of the hydrophobic mineral in the floated fraction. The values of air flow used in this work were transformed into superficial air rate, which is defined as being the relation between air flow and the area of the transversal section of the column, under normal conditions of temperature (21˚C) and pressure (147 psi). Tests were carried out by varying the superficial air rate from 1.25 to 2.00 cm/s with an average solid feed ratio of 24.7 kg/h, as depicted in Figure 6. It can be observed that, within the levels studied, the rise in the superficial air rate resulted in a reduction of Fe recovery, with the rise in the SiO2 content in the concentrate.
4. Conclusions
The iron ore refuse emerging from the classification and pre-concentration phases, in the minor mining companies in the Quadrilatero Ferrifero region, present a great potential of being used through column flotation.
CMC (carboxymethylcellulose) presented fair selectivity in the separation of iron minerals and silicates, slightly higher than that of corn starch; due to the cost/benefit equation, corn starch is the most indicated.
The superficial air rate of 1.25 and 1.50 cm/s presented satisfactory results in the flotation of the samples studied.
Although tests were carried out in rougher-cleaner and rougher-scavenger, satisfactory results were achieved with a circuit of only one rougher flotation column, where a concentrate with Fe over 66.0% and SiO2 below 1.0% were obtained. Metal and mass recoveries were satisfactory as well.
5. Acknowledgements
The authors should like to thank FAPEMIG, Institution Research Support of the State of Minas Gerais—Brazil, for the support rendered in the form of financial fund, without which the present work would have never been done.
REFERENCES
- R. Houot, “Beneficiation of Iron Ore by Flotation—Review of Industrial and Potential Applications,” International Journal of Mineral Processing, Vol. 10, No. 3, 1983, pp. 183-204. doi:10.1016/0301-7516(83)90010-8
- I. M. Flint, H. E. Wyslouzil, V. L. L. Andrade and D. J. Murdoch, “Column Flotation of Iron Ore,” Minerals Engineering, Vol. 5, No. 10-12, 1992, pp. 1185-1194. doi:10.1016/0892-6875(92)90158-6
- A. C. Araujo and A. E. C. Peres, “Froth Flotation: Relevant Facts and the Brazilian Case,” Série Tecnologia Mineral, CETEM, Rio de Janeiro, 1995, pp. 70-78.
- M. B. M. Monte and A. E. C. Peres, “Química de Superfí- cie na Flotação,” In: A. Luz, Ed., Tratamento de Minérios, 3rd Edition, Rio de Janeiro, 2002, pp. 339-407.
- A. C. Araujo and P. R. M. Viana, “Minérios de Ferro e Seus Métodos de Concentração IV Simpósio Brasileiro de Minério de Ferro,” Belo Horizonte, 2003, pp. 750-758.
- I. Iwasaki, “Iron Ore Flotation, Theory and Practice,” In: P. S. Mapa, Ed., Mining Engineering, M.Sc Dissertation, UFMG, 2006, pp. 26-28.
- P. J. B. Rabelo, “Estudos de Caracterização e Redução do Teor De Fósforo do Minério de Ferro da Mina de Alegria, Mariana, MG,” M.Sc Dissertation, UFMG, 1994, pp. 23- 25.

