Surgical Science
Vol.06 No.07(2015), Article ID:57733,9 pages
10.4236/ss.2015.67042
SImmetry® Sacroiliac Joint Fusion System with SImmetry Decorticator®
Brian Beaubien1, Richard M. Salib2, Louis C. Fielding3, Jon E. Block3*
1Zyga Technology, Inc., Minnetonka, USA
2Institute for Low Back and Neck Care, Minneapolis, USA
3The Jon Block Group, San Francisco, USA
Email: *jonblock@jonblockgroup.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 May 2015; accepted 30 June 2015; published 3 July 2015
ABSTRACT
Low back pain (LBP) is one of the most prevalent, disabling and costly medical conditions affecting modern society. LBP presents a significant challenge to effective treatment due to often multifactorial or unknown etiology. Since the 1980s, the sacroiliac (SI) joint has become increasingly recognized as a common source of LBP. In contrast to other sources of LBP such as internal disc disruption and even psychosocial factors, SI joint pain and degeneration are reliably identified with provocative manual tests and diagnostic injections. Fusion of the SI joint has been shown to provide enduring symptom relief, and minimally invasive techniques developed over the past decade have further reduced the operative risks associated with open fusion surgery. Minimally invasive SI joint fusion surgeries are typically performed by placing rigid implant components across the joint space. The implants provide mechanical fixation while bony fusion develops. Decortication of the SI joint space during the procedure produces a bleeding bone surface that allows for increased availability of autologous mesenchymal stem cells and growth factors at the fusion site. Coupled with the mechanical stability provided by the implant and autologous bone graft, decortication provides an optimal environment for bone growth and subsequent fusion of the joint. This report describes the background of SI joint disease, treatment, and the minimally invasive SImmetry® Sacroiliac Joint Fusion System (Zyga Technology, Inc., Minnetonka, MN, USA), with emphasis on the decortication instrumentation and procedure.
Keywords:
Sacroiliac, Decortication, Low Back Pain, Minimally Invasive

1. Introduction
1.1. Background and Prevalence of Sacroiliac Joint Disease
Low back pain is a highly prevalent and costly condition: it affects 70% - 85% of all people at some time in life, with prevalence between 75 and 100 per 1000 population among all age groups above 18 years [1] . Approximately 2% of the working population in the United States receives compensation for back injuries each year [1] . Back pain is particularly challenging to physicians, as the pain is often nonspecific and may originate from the spine (including the intervertebral disc, nerve roots and facet joints), hip, sacroiliac (SI) joints, or psychosocial factors [2] [3] . Over the past century, and increasingly in recent decades, the SI joint has been recognized as a common source of low back pain, likely representing 15% - 30% of patients with axial low back pain [2] [4] -[6] . The burden of SI joint disease is significant. The prevalence in the US is estimated at up to 10 million, and the impact is severe, with an average calculated loss of 0.5 QALYs (quality-adjusted life years); the patient burden of SI joint pain is similar to other significant orthopedic conditions (e.g., hip or knee osteoarthritis, spinal stenosis, and degenerative spondylolisthesis), and equivalent to that of chronic depression or severe chronic obstructive pulmonary disease (COPD) [7] .
1.2. Sacroiliac Joint Anatomy
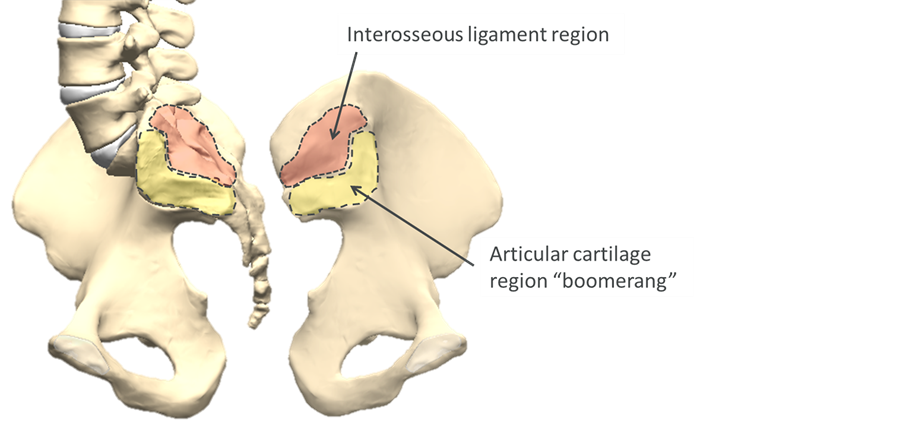
The SI joint (Figure 1) is complex, with highly variable individual anatomy. Historically, the SI joint has been described variably as a mobile diarthrodial joint, ossified synarthrodial joint, and an intermediate “amphiarthrosis” [8] . The ventral portion of the joint has articular cartilage and synovial fluid. The sacral surface is concave and the iliac portion is convex. The dorsal portion comprises a ligamentous structure with large variability in surface features and joint gap [9] . The auricular portion has an irregular “boomerang” shape, including cartilaginous ridges that are considered adaptive to the loading environment [10] . Even in symptomatic patients, total motion of the joint has been found to be small, typically 1˚ - 2˚ rotation and 5 mm translation between supine and sitting or standing [11] . There is significant dimorphism among the sexes, with males having a larger, more irregularly shaped articular surface and approximately 40% less mobility, likely due to both the differences in loading and the demands of childbirth [8] . The SI joint is highly innervated by the lumbosacral nerve roots, including both nociceptive and proprioceptive mechanoreceptors [12] .
1.3. Pathology
SI joint pain may result from several different sources such as direct or iatrogenic trauma, pelvic shear, childbirth, degenerative or inflammatory processes, or be idiopathic in nature [12] . The SI joint is subject to age-re-
Figure 1. Sacroiliac jointanatomy; dis-articulated view of the sacral surface of the joint (left) and iliac surface (right).
lated degeneration, including intra and extra-articular osteophytosis and ankyloses, which is typically associated with increased motion of the joint [8] . The ventral joint capsule is relatively thin, allowing for synovial leaks [8] . Childbirth is particularly stressful to the SI joints: multiparous women have been found to have significantly greater intrapelvic motion compared with men or nulliparous women [8] , with pelvic and SI joint pain a common sequel [13] . Iatrogenic injury to the SI joint is also a common result of spinal fusion, due to both the harvest of iliac crest bone graft (ICBG) and the added mechanical stresses placed on the joint by spinal fusion [5] [14] - [17] , resulting in SI joint pain in up to 43% of patients after lumbar or lumbosacral fusion [18] . The proximity of the SI joint to the piriformis muscle and abundant innervation may provoke sciatic irritation and referred pain to the buttock, calf and foot, mimicking radiculopathy [19] [20] .
1.4. Diagnosis
The SI joints have complex and multi-planar morphology that challenges traditional radiography [9] . While plain films, computed tomography (CT) and magnetic resonance imaging (MRI) may identify SI joint abnormalities, there are no definitive findings that are reliably associated with painful SI joints [12] . The present gold standards for diagnosis of pain originating in the SI joints are manual provocative tests and controlled anesthetic diagnostic injections. Provocative manual tests include the distraction, thigh thrust, compression, flexion-ab- duction-external rotation (FABER) and Gaenslen tests. In a systematic review, Szadek et al. found that three or more positive stressing tests, including the thigh thrust test, were typically required to diagnose SI joint pain [21] . Diagnostic injections are typically performed following the noninvasive provocative tests. Comparative and controlled local anesthetic blocks have been found to have good diagnostic utility for identifying SI joint pain [12] [22] and confirming the provocative tests.
1.5. Treatment
Conservative care for SI joint pain may include analgesics, physical therapy or spinal manipulation therapy. There is poor evidence for the effectiveness of therapeutic injections [12] [22] [23] . Fusion of the SI joint was introduced by Smith-Petersen in 1921 [24] , with instrumented fixation becoming more common since the 1980s [25] [26] . Open SI joint fusion is a highly invasive procedure, typically involving open exposure of the joint, graft harvesting and bone bed preparation, and either instrumentation such as plates and screws or a prolonged period of postoperative immobilization. Operative morbidities of open fusion procedures are high [26] , and clinical results have been mixed [27] .
Over the past decade, minimally invasive SI joint fusion systems have been developed to achieve the pain relief and clinical benefit of a successful fusion, while lowering the surgical morbidity associated with open SI joint fusion procedures. Minimally invasive surgery (MIS) procedures have shown significantly improved operative results compared to open techniques [28] ; however, non-union remains a persistent challenge [29] [30] . All MIS systems provide acute fixation of the SI joint, whether through the use of screws, cannulated threaded cages, or impacted components; some systems additionally provide space for placement of bone graft within and surrounding the implants. The SImmetry Sacroiliac Joint Fusion System with SImmetry Decorticator (Zyga Technology, Inc., Minnetonka, MN, USA) builds upon the operative advantages of MIS SI joint fusion systems, with the addition of proprietary decortication instruments for preparation of the fusion site and improved, larger interosseous fusion.
1.6. Role of Decortication in Arthrodesis
Both mechanical and biological conditions are required for successful arthrodesis. Pluripotent mesenchymal stem cells originating in hemopoetic marrow differentiate into skeletal tissue such as cartilage, fibrous tissue or bone depending on these factors, as described by Carter [31] . As illustrated in Figure 2, predominant tensile stress will result in fibrous tissue while excessive compressive stress results in cartilage. Lower stress regions may develop either bone or cartilage, depending on whether vascularity is good or poor, respectively. In the example of fracture healing, fixation is responsible for creating the mechanical conditions for healing bone, while the hematoma and callous maintain the necessary biological environment provided by the vascularity, stem cells and growth factors originating in the subchondral bone and marrow. In orthopaedic practice, the most commonly achieved bony union is fracture repair through immobilization using internal (e.g. plate or rod) or
Figure 2. Roles of mechanical stresses and vascularity on tissue differentiation (adapted from Carter [31] ).
external fixation (e.g. external fixatorsor casting). The trauma at the fracture site provides the initial bleeding, hematoma and callus formation that naturally create the biological environment conducive to healing and bone formation. In arthrodesis surgery these conditions must be created surgically, typically through cartilaginous debridement and decortication of the joint space.
As described above, the SI joint has been considered to be an amphiarthrosis. Continuing the fracture healing analogy, a painful, degenerative SI joint bears many similarities to an unhealed pseudarthrosis, where residual motion and the biological environment result in a chronically painful and unstable condition that requires true arthrodesis to resolve.
Decortication prior to placement of autologous bone graft in spinal fusion was first described by Hibbs in 1924 [32] . Decortication has since been considered a critical component of intervertebral fusion, as the exposed marrow, bleeding and hematoma provide the mesenchymal stem cells, osteoinductive proteins and local inflammatory response that facilitate bone formation and fusion [33] [34] . In a study of vascularization of inter- transverse process spinal fusion, Toribatake et al. found that the primary interosseous blood supply for vascularization of the graft came from the decorticated transverse processes, and that there was significantly less vascularization where nonunion or cartilaginous fusion masses occurred [35] . Comparing spinal fusion with or without decortication and/or instrumentation, Ishikawa et al. found that in both instrumented and non-instrumented groups, decorticated specimens had more new bone with no clear demarcation between bone and graft, while the non-decorticated specimens in both groups had less bone growth and displayed a fibrocartilaginous zone at the graft incorporation boundary [36] . These findings clearly demonstrate that decortication provides conditions favorable to development of a solid union that may not be achievable with instrumentation alone. Toribatake’s and Ishikawa’s findings are consistent with the model of skeletal tissue differentiation and regeneration described by Carter, where both mechanical and biological factors are critical to determining tissue differentiation and ultimate fusion. Successful arthrodesis of the SI joints utilizes the same processes. Fixation provides the required mechanical conditions, while decortication and placement of autograft provide the biological environment favorable to a strong union.
2. SImmetry Sacroiliac Joint Fusion System with SImmetry Decorticator
2.1. Implant System and Surgical Technique
The SImmetry Sacroiliac Joint Fusion System implant is illustrated below in Figure 3. The implant system
Figure 3. SImmetry Sacroiliac Joint Fusion System Implant with decorticated fusion regions (red discs). Sagittal view (left) and coronal view (right). (A) 12.5 diameter cannulated implants; (B) 8.5 mm anti-rotation implants.
comprises large 12.5 mm diameter cannulated implants at the fusion sites, as well as 8.5 mm diameter anti-rota- tion implants that provide additional mechanical stability during the development of arthrodesis. Unlike other MIS SI joint fusion implants that are impacted across or between the joint surfaces, the SImmetry implants are characterized by deep corticocancellous threads that provide mechanical fixation and stability without distraction of the articular surfaces. The threaded surfaces of the implants are prepared with a 2 - 4 μm surface roughness that has been shown to be optimal for osseointegration [37] [38] without the delamination during insertion, ongrowth or removal that has been associated with hydroxyapatite-coated or titanium plasma-sprayed surfaces [39] . Additionally illustrated in Figure 3 are the large decortication areas surrounding the 12.5 mm diameter cannulated implants.
The four principal steps of the surgical procedure are minimally invasive lateral access, joint preparation, bone graft placement, and implant delivery. After a 1.5 - 2 cm incision is made, a dilator and guide pin are used to access the ilium and SI joint under fluoroscopic guidance. Acannulated drill is then advanced over the guide pin to create an osseous tunnel through the ilium. Ilium drillings are collected for use in the bone graft, avoiding the need for direct ICBG harvest. The joint is prepared as shown in Figure 4, removing cartilage and utilizing the unique, flexible SImmetry Decorticator to decorticate the joint area surrounding the tunnel, while irrigation and suction are used to extract joint tissue. A series of three sequentially larger Decorticators (described further below) are used to prepare the fusion bed and graft cavity in approximately 5 minutes. Approximately 5 cc bone graft, including autologous bone from the ilium drillings, is then packed into the prepared cavity to promote solid arthrodesis.
Fixation is then achieved with at least one 12.5 mm diameter cannulated implant, and one or more additional implants to ensure rotational stability. Typically an 8.5 mm implant is placed just superior to the 12.5 mm implant, but may also be placed inferiorly. Final fluoroscopic images are obtained to confirm correct placement, and the deep tissues and skin incision may be infiltrated with bupivacaine and epinephrine for postoperative pain control. Patients may be discharged the same day, or admitted to a 1 - 2 day hospital stay for pain control, with pain medication as needed. Fusion status is typically assessed with CT12 months after the procedure.
2.2. SImmetry Decorticator
Unique among minimally invasive SI joint fusion systems, the SImmetry Decorticator provides a simple means to decorticate the fusion bed, creating the optimal biological conditions for solid arthrodesisvia a minimally invasive, lateral approach. The decorticators, illustrated in Figure 5, include a handle with a profile mark for visual
Figure 4. Cartilage removal and decortication with simmetry Decorticator. (A) flexible cutting element advanced into joint space; (B) cartilage removed and surface of ilium decorticated; (C) radiographic view of decorticator with cutting element extended into joint space; (D) oblique lateral view of decorticated joint (inset: radiographic view).
Figure 5. SImmetry decorticator. Complete instrument (top); detailed views of flexible cutting elements of decorticators 1, 2 and 3 (bottom).
orientation of the instrument; cutting element with integral guide ribbon; knob for incremental deployment of the cutting element; and depth stop for optional control of decorticator depth. Three sequentially larger decorticators are utilized to allow for a large graft volume of 2.1 cm3. The intuitive design of the decorticators allows joint preparation to be achieved inapproximately 5 minutes via a simple, lateral approach that is perpendicular to the joint surface. The lateral approach preserves the peri-articular structures, thus avoiding further iatrogenic destabilization of the joint.
The flexible distal end of the decorticator includes a guide ribbon and cutting element that extend beyond the margins of the tunnel. The integral guide ribbon is able to follow the undulating surface of the SI joint with the cutting element denuding cartilage and decorticating the surface of the fusion site. This enables the decorticator to access and prepare approximately 5 cm2 of the ilial and sacral surfaces beyond the implant, representing up to 50% of the area of the auricular joint surface [40] , as shown in Figure 6. The flexible cutting element decorticates the surface of the joint for optimal fusion conditions, without removing all cortical bone, such that the cortico-cancellous interface is preserved.
When the prepared joint area is packed with bone graft and ultimately secured with the threaded implants, optimal conditions for intra-articular fusion are achieved. The decorticated subchondral bone and hemopoietic marrow provide the initial blood, mesenchymal stem cells, and ultimate vascularization that lead to strong, bony
Figure 6. Cadaveric specimens prepared with simmetry decorticator.
fusion [33] -[36] . Ample, precisely placed bone graft provides an osteoconductive matrix, and finally secure fixation with the threaded implants creates a biomechanically stable construct to provide the conditions necessary for osteosynthesis and solid arthrodesis.
3. Discussion
Chronic low back pain is a highly prevalent, disabling condition with enormous cost to society. Of the multiple possible underlying etiologies responsible for low back pain, the SI joint is recognized as a significant source. Chronic SI joint pain is a disabling condition with significant impact on patient quality of life. The symptoms often resemble axial low back pain from other sources as well as radiculopathy, however a combination of manual provocative tests and controlled diagnostic injections have led to a reliable algorithm for differential diagnosis of pain originating in the SI joint.
The anatomy of the SI joint is complex, and has been described as an amphiarthrosis due to having both diarthrodial and synarthrodial features. The joint is subject to classic age-related degeneration as well as trauma from childbirth and iatrogenic injury following lumbar fusion. Studies of degenerative SI joints reveal increased articular motion of the pathological joint. The result is a chronic, painful condition analogous to a pseudarthrosis, where the combination of poor vascularity and instability create biological and biomechanical conditions that perpetuate the situation. Local pain may originate from the chronic, degenerative inflammatory response in this highly innervated, complex muscular region; sciatic irritation may result in pain referred to the buttock, calf and foot.
Fusion of the SI joint has long been established as the ultimate solution for patients who have failed conservative therapy. Arthrodesis accompanied by decortication, bone grafting and mechanical fixation creates the biological and biomechanical conditions for intra-articular bone growth, restabilizing the joint to break the pattern of chronic instability and pain. Open surgical SI joint fusion as performed throughout the 20th century provides symptom resolution when successful. However, open fusion is a highly invasive procedure, with significant operative morbidity associated with surgical access, graft harvesting and placement.
Minimally invasive SI joint fusion has become increasingly popular over the past decade. These procedures significantly reduce the surgical time and complications that plagued open fusion. Minimally invasive procedures typically compromise with minimal access to the joint area, limiting the amount of decortication and graft material able to be deposited at the intended fusion site. If insufficient joint area is biologically and biomechanically available for proper incorporation of the graft and fusion mass development, the construct will largely rely upon the mechanical fixation provided solely by the minimally invasive implants. Over time, these may loosen, with a return to the chronically unstable, painful condition that the fusion was intended to address.
The SImmetry Sacroiliac Joint Fusion System addresses these issues both with the design of the implants, and particularly with the unique decortication technique utilizing the SImmetry Decorticator. The decorticator enables the surgeon to remove articular cartilage and decorticate 5 cm2 of the SI joint surrounding the 12.5 mm diameter cannulated implant. This prepared area, representing up to 50% of the auricular joint surface, ensures ample bioavailability of pluripotent mesenchymal stem cells, growth factors and vascularity from the subchondral bone and marrow. With the prepared area packed with bone graft and stabilized with the threaded implants, optimal conditions for a solid fusion are established. Autologous blood containing stem cells and growth factors proliferates into the osteoconductive graft material. The mechanical fixation maintains the proper stress environment, and vascular supply from the subchondral bone and marrow allow for the stem cells to differentiate into woven bone rather than the fibrocartilagenous tissue that would result without these necessary conditions.
4. Conclusion
Pain originating in the SI joint is a highly prevalent and disabling condition. When SI joint disease is positively diagnosed and refractory to conservative care, fusion of the joint is indicated. Open surgical fusion may be effective, yet has major operative risks and morbidities. While minimally invasive SI joint fusion techniques greatly improve upon the disadvantages of open fusion, this typically comes at a cost of minimal joint preparation, resulting in a much smaller fusion mass and increased risk of nonunion. The SImmetry Sacroiliac Joint Fusion System builds upon the operative advantages of a minimally invasive approach, with the addition of aninnovative decortication technique that allows preparation of up to 50% of the auricular surface of the SI joint. The decorticated subchondral bone and marrow provide a vascular environment rich in growth factors and mesenchymal stem cells that, when combined with the rigid fixation provided by the threaded implants, create an optimal environment for development of a solid fusion.
Acknowledgements
Brian Beaubien is an employee of Zyga Technology, Inc. (Minnetonka, MN, USA). Jon Bock Group Authors received financial support from Zyga Technology. Figure 1 and Figures 3-6 were provided by Zyga Technology, Inc.
References
- Andersson, G.B. (1999) Epidemiological Features of Chronic Low-Back Pain. Lancet, 354, 581-585. http://dx.doi.org/10.1016/S0140-6736(99)01312-4
- Sembrano, J.N. and Polly Jr., D.W. (2009) How Often Is Low Back Pain Not Coming from the Back? Spine, 34, E27- E32. http://dx.doi.org/10.1097/BRS.0b013e31818b8882
- Derebery, V.J. and Tullis, W.H. (1986) Low Back Pain Exacerbated by Psychosocial Factors. Western Journal of Medicine, 144, 574-579.
- Cohen, S.P. (2005) Sacroiliac Joint Pain: A Comprehensive Review of Anatomy, Diagnosis, and Treatment. Anesthesia & Analgesia, 101, 1440-1453. http://dx.doi.org/10.1213/01.ANE.0000180831.60169.EA
- Maigne, J.Y. and Planchon, C.A. (2005) Sacroiliac Joint Pain after Lumbar Fusion. A Study with Anesthetic Blocks. European Spine Journal, 14, 654-658. http://dx.doi.org/10.1007/s00586-004-0692-6
- Schwarzer, A.C., Aprill, C.N. and Bogduk, N. (1995) The Sacroiliac Joint in Chronic Low Back Pain. Spine, 20, 31-37.
- Cher, D., Polly, D. and Berven, S. (2014) Sacroiliac Joint Pain: Burden of Disease. Medical Devices: Evidence and Research, 7, 73-81. http://dx.doi.org/10.2147/MDER.S59437
- Vleeming, A., Schuenke, M.D., Masi, A.T., et al. (2012) The Sacroiliac Joint: An Overview of Its Anatomy, Function and Potential Clinical Implications. Journal of Anatomy, 221, 537-567. http://dx.doi.org/10.1111/j.1469-7580.2012.01564.x
- Ebraheim, N.A., Mekhail, A.O., Wiley, W.F., Jackson, W.T. and Yeasting, R.A. (1997) Radiology of the Sacroiliac Joint. Spine, 22, 869-876. http://dx.doi.org/10.1097/00007632-199704150-00009
- Vleeming, A., Volkers, A.C., Snijders, C.J. and Stoeckart, R. (1990) Relation between Form and Function in the Sacroiliac Joint. Part II: Biomechanical Aspects. Spine, 15, 133-136. http://dx.doi.org/10.1097/00007632-199002000-00017
- Sturesson, B., Selvik, G. and Uden, A. (1989) Movements of the Sacroiliac Joints. A Roentgen Stereophotogrammetric Analysis. Spine, 14, 162-165. http://dx.doi.org/10.1097/00007632-198902000-00004
- Hansen, H.C., McKenzie-Brown, A.M., Cohen, S.P., Swicegood, J.R., Colson, J.D. and Manchikanti, L. (2007) Sacroiliac Joint Interventions: A Systematic Review. Pain Physician, 10, 165-184.
- Mens, J.M., Vleeming, A., Stoeckart, R., Stam, H.J. and Snijders, C.J. (1996) Understanding Peripartum Pelvic Pain. Implications of a Patient Survey. Spine, 21, 1363-1369; Discussion 1369-1370. http://dx.doi.org/10.1097/00007632-199606010-00017
- Ebraheim, N.A., Elgafy, H. and Semaan, H.B. (2000) Computed Tomographic Findings in Patients with Persistent Sacroiliac Pain after Posterior Iliac Graft Harvesting. Spine, 25, 2047-2051. http://dx.doi.org/10.1097/00007632-200008150-00008
- Ha, K.Y., Lee, J.S. and Kim, K.W. (2008) Degeneration of Sacroiliac Joint after Instrumented Lumbar or Lumbosacral Fusion: A Prospective Cohort Study over Five-Year Follow-Up. Spine, 33, 1192-1198. http://dx.doi.org/10.1097/BRS.0b013e318170fd35
- Ivanov, A.A., Kiapour, A., Ebraheim, N.A. and Goel, V. (2009) Lumbar Fusion Leads to Increases in Angular Motion and Stress across Sacroiliac Joint: A Finite Element Study. Spine, 34, E162-E169. http://dx.doi.org/10.1097/BRS.0b013e3181978ea3
- Kurz, L.T., Garfin, S.R. and Booth Jr., R.E. (1989) Harvesting Autogenous Iliac Bone Grafts. A Review of Complications and Techniques. Spine, 14, 1324-1331. http://dx.doi.org/10.1097/00007632-198912000-00009
- DePalma, M.J., Ketchum, J.M. and Saullo, T.R. (2011) Etiology of Chronic Low Back Pain in Patients Having Undergone Lumbar Fusion. Pain Medicine, 12, 732-739. http://dx.doi.org/10.1111/j.1526-4637.2011.01098.x
- Weksler, N., Velan, G.J., Semionov, M., Gurevitch, B., Klein, M., Rozentsveig, V. and Rudich, T. (2007) The Role of Sacroiliac Joint Dysfunction in the Genesis of Low Back Pain: The Obvious Is Not Always Right. Archives of Orthopaedic and Trauma Surgery, 127, 885-888. http://dx.doi.org/10.1007/s00402-007-0420-x
- Chen, Y.C., Fredericson, M. and Smuck, M. (2002) Sacroiliac Joint Pain Syndrome in Active Patients: A Look behind the Pain. The Physician and Sportsmedicine, 30, 30-37. http://dx.doi.org/10.3810/psm.2002.11.527
- Szadek, K.M., van der Wurff, P., van Tulder, M.W., Zuurmond, W.W. and Perez, R.S. (2009) Diagnostic Validity of Criteria for Sacroiliac Joint Pain: A Systematic Review. The Journal of Pain, 10, 354-368. http://dx.doi.org/10.1016/j.jpain.2008.09.014
- Manchikanti, L., Abdi, S., Atluri, S., Benyamin, R.M., Boswell, M.V., Buenaventura, R.M., et al. (2013) An Update of Comprehensive Evidence-Based Guidelines for Interventional Techniques in Chronic Spinal Pain. Part II: Guidance and Recommendations. Pain Physician, 16, S49-S283.
- Chou, R., Loeser, J.D., Owens, D.K., Rosenquist, R.W., Atlas, S.J., Baisden, J., et al. (2009) Interventional Therapies, Surgery, and Interdisciplinary Rehabilitation for Low Back Pain: An Evidence-Based Clinical Practice Guideline from the American Pain Society. Spine, 34, 1066-1077. http://dx.doi.org/10.1097/BRS.0b013e3181a1390d
- Smith-Petersen, M.N. (1921) Arthrodesis of the Sacroiliac Joint. A New Method of Approach. Journal of Bone and Joint Surgery, 3, 400-405.
- Rand, J.A. (1985) Anterior Sacro-Iliac Arthrodesis for Post-Traumatic Sacro-Iliac Arthritis. A Case Report. Journal of Bone and Joint Surgery, 67, 157-159.
- Buchowski, J.M., Kebaish, K.M., Sinkov, V., Cohen, D.B., Sieber, A.N. and Kostuik, J.P. (2005) Functional and Radiographic Outcome of Sacroiliac Arthrodesis for the Disorders of the Sacroiliac Joint. The Spine Journal, 5, 520-528; Discussion 529. http://dx.doi.org/10.1016/j.spinee.2005.02.022
- Schutz, U. and Grob, D. (2006) Poor Outcome Following Bilateral Sacroiliac Joint Fusion for Degenerative Sacroiliac Joint Syndrome. Acta orthopaedica Belgica, 72, 296-308.
- Smith, A.G., Capobianco, R., Cher, D., Rudolf, L., Sachs, D., Gundanna, M., et al. (2013) Open versus Minimally Invasive Sacroiliac Joint Fusion: A Multi-Center Comparison of Perioperative Measures and Clinical Outcomes. Annals of Surgical Innovation and Research, 7, 14. http://dx.doi.org/10.1186/1750-1164-7-14
- Ashman, B., Norvell, D.C. and Hermsmeyer, J.T. (2010) Chronic Sacroiliac Joint Pain: Fusion versus Denervation as Treatment Options. Evidence-Based Spine-Care Journal, 1, 35-44. http://dx.doi.org/10.1055/s-0030-1267066
- Rudolf, L. and Capobianco, R. (2014) Five-Year Clinical and Radiographic Outcomes after Minimally Invasive Sacroiliac Joint Fusion Using Triangular Implants. The Open Orthopaedics Journal, 8, 375-383. http://dx.doi.org/10.2174/1874325001408010375
- Carter, D.R. (1987) Mechanical Loading History and Skeletal Biology. Journal of Biomechanics, 20, 1095-1109. http://dx.doi.org/10.1016/0021-9290(87)90027-3
- Hibbs, R.A. (1988) A Report of Fifty-Nine Cases of Scoliosis Treated by the Fusion Operation. By Russell A. Hibbs, 1924. Clinical Orthopaedics and Related Research, 229, 4-19.
- Friedenstein, A.J., Petrakova, K.V., Kurolesova, A.I. and Frolova, G.P. (1968) Heterotopic Transplant of Bone Marrow. Analysis of Precursor Cells for Osteogenic and Hematopoietic Tissues. Transplantation, 6, 230-247. http://dx.doi.org/10.1097/00007890-196803000-00009
- Muschler, G.F., Lane, J.M. and Dawson, E.G. (1990) The Biology of Spinal Fusion. In: Cotler, J.M. and Cotler, H.B., Eds., Spinal Fusion: Science and Technique, Springer-Verlag, New York, 9-21. http://dx.doi.org/10.1007/978-1-4612-3272-8_2
- Toribatake, Y., Hutton, W.C., Tomita, K. and Boden, S.D. (1998) Vascularization of the Fusion Mass in a Posterolateral Intertransverse Process Fusion. Spine, 23, 1149-1154. http://dx.doi.org/10.1097/00007632-199805150-00015
- Ishikawa, S., Shin, H.D., Bowen, J.R. and Cummings, R.J. (1994) Is It Necessary to Decorticate Segmentally Instrumented Spines to Achieve Fusion? Spine, 19, 1686-1690. http://dx.doi.org/10.1097/00007632-199408000-00006
- Wennerberg, A. and Albrektsson, T. (2009) Effects of Titanium Surface Topography on Bone Integration: A Systematic Review. Clinical Oral Implants Research, 20, 172-184. http://dx.doi.org/10.1111/j.1600-0501.2009.01775.x
- Schwartz, Z., Raz, P., Zhao, G., Barak, Y., Tauber, M., Yao, H. and Boyan, B.D. (2008) Effect of Micrometer-Scale Roughness of the Surface of Ti6Al4V Pedicle Screws in Vitro and in Vivo. The Journal of Bone and Joint Surgery, 90, 2485-2498. http://dx.doi.org/10.2106/JBJS.G.00499
- Franchi, M., Orsini, E., Martini, D., Ottani, V., Fini, M., Giavaresi, G., et al. (2007) Destination of Titanium Particles Detached from Titanium Plasma Sprayed Implants. Micron, 38, 618-625. http://dx.doi.org/10.1016/j.micron.2006.09.005
- Mahato, N.K. (2010) Variable Positions of the Sacral Auricular Surface: Classification and Importance. Neurosurgical Focus, 28, E12. http://dx.doi.org/10.3171/2009.12.focus09265
NOTES

*Corresponding author.