Open Journal of Obstetrics and Gynecology
Vol.07 No.06(2017), Article ID:77277,10 pages
10.4236/ojog.2017.76066
Unilateral Singleton Pregnancy with Rural Vaginal Delivery in a Woman with Uterus Didelphys
Justin Desrochers, Kalun Boudreau, Adewale Adegbenro
Department of Medicine, University of British Colombia, Vancouver, Canada
Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 21, 2017; Accepted: June 25, 2017; Published: June 28, 2017
ABSTRACT
Background: Uterus didelphys is a Müllerian duct anomaly which is clinically significant because only 45% of UD patients achieve term delivery and have associated increased risk of spontaneous abortion, foetal growth retardation, mal presentation, and a significant caesarean section delivery rate. Case: A 26-year-old Gravida 2 Para 1 Abortion 1 woman with uterus didelphys and associated complete, non-communicating, longitudinal vaginal septum carries a pregnancy to term in her right uterus in rural Canada. She delivers her baby at 41 weeks gestational age via vacuum assisted spontaneous vaginal delivery. Conclusion: This case report supports more recent literature that uterus didelphys should not be considered as an absolute indication for caesarean delivery. A trial of labour is conceivable with obstetrician gynaecologist involvement in more rural centers with a care plan in place.
Keywords:
Pregnancy, Delivery, Uterus Didelphys, Didelphys Uterus, Müllerian Duct Anomaly

1. Introduction
Uterus Didelphys (UD) is a Class III Müllerian duct anomaly (MDA)resulting in a double uterus, double cervix and in most cases a longitudinal vaginal septum ranging from thin and easily displaced to thick and inelastic [1] . A longitudinal vaginal septum is associated with 75% of these anomalies and no communication is present between the two endometrial cavities and the two horns [2] [3] [4] [5] . The remaining MDAs include: Class I―vaginal agenesis, Class II―un- icornuate, Class IV―bicornuate, Class V―septate, and Class VI―arcuate. The Müllerian ducts give rise to the following structures: fallopian tubes, uterus, the cervix, and the proximal two thirds of the vagina [3] . MDAs are multifactorial, resulting from a spectrum of improper development, fusion, canalization, or reabsorption of the Müllerian ducts between 6 and 20 weeks gestation [3] [4] . Since the Müllerian ducts develop in close association with the Wolffian ducts, renal anomalies can be found in association with MDAs [2] . The incidence of MDAs is estimated to range from 0.5% - 5%, while the prevalence ranges from 5.5% - 9.8% in the general population [2] [3] [6] . UD results from nearly complete failure in fusion of the Müllerian ducts and remains the second least common type of MDA, comprising 5% - 8.3% of all MDAs [2] [4] .
Most women with UD are asymptomatic and diagnosis is often delayed [7] . The diagnosis becomes apparent when patients present with concerns of infertility, dyspareunia, dysmenorrhea, leukorrhea often in association with a thick partial or complete obstructing vaginal septum on examination [2] [3] [7] . MRI and the developing three-dimensional ultrasound are currently considered as the best imaging modalities for detecting and classifying MDA’s [5] [8] [9] [10] [11] . Traditionally, accurate diagnosis of UD has most reliably accomplished with more invasive laparoscopy and hysteroscopy [12] .
Menses is most often regular in these women [2] . The obstructing septum can produce a complete or incomplete obstructive hemivagina [1] . Upon menarche, this can lead to an accumulation of menstrual blood in the vagina (hematocolpos) and uterus (hematometrocolpos), presenting as chronic abdominal pain [1] [2] . In addition, outside of facilitating vaginal delivery, longitudinal septum excision is only indicated for symptomatic woman, most commonly dyspareunia or obstructing septum producing painful hematometrocolpos [2] . Despite these potential obstructive symptoms, there is no increased incidence of endometriosis or gynaecological neoplasm in these patients [1] . Finally, renal anomalies should always be investigated in all UD patients to rule out Hernyl-Werner-Wunderlich syndrome (rare congenital anomaly characterised by UD blind hemivagina and ipsilateral renal agenesis) [13] [14] .
The impact of UD on fertility is divided in the literature. Interestingly, twin pregnancies have been described to occur more frequently in UD, with up to seven times the usual incidence [15] . Some sources suggest fertility consultation and treatment with others cite no need for alterations in a pregnancy care plan for a patient with UD [1] [6] [7] [16] [17] . There is insufficient data to suggest metrorplasty in fertility concerns related to UD unless all other avenues have been explored [1] [2] [7] [12] [18] . Multiple pregnancies are described in the literature but are not common in UD [19] [20] [21] [22] [23] .
Seeing as cervical incompetence occurs in up to 30% of all MDAs, it has been suggested to screen for and assess the need for cerclage in all pregnant patients with an MDA [18] [24] [25] [26] . However, in contrast to other MDAs, UD is not as strongly associated with cervical incompetence, and as such, other sources have recommended against routine screening unless there is a personal history of incompetence [1] [2] [27] [28] . Cerclage is best considered in UD when there is a history of recurrent miscarriages and if premature dilation is identified in the antepartum period [2] [12] .
UD has generally been found to have better pregnancy outcome than other MDAs [1] . Commonly cited associations with UD include increased risk of spontaneous abortion, foetal growth retardation, premature labour with a 45% term delivery rate, increased caesarean section (C/S) delivery rates due to breech presentation, decreased live births and variably cervical incompetency [1] [6] [7] [16] [24] [29] [30] . Due to these complications, UD patients belong to a higher- risk group and deserve meticulous prenatal care [7] .
Herein, we add to the discussion of vaginal delivery of women with UD with a non-communicating longitudinal vaginal septum and the possibility to handle such higher risk cases in a rural centre.
2. Case Presentation
This patient is a 26-year-old G2T0P0A1L0 Caucasian woman of 63.5 kg (change this to BMI) with known UD and a complete longitudinal vaginal septum. She is the current resident of rural community of 1000 in rural British Colombia, Canada. After the onset of menses, she noticed that she would have vaginal bleeding despite having inserted a tampon. Subsequently she noted that she had two sides to her vagina with a dividing septum. Her MDA was officially diagnosed as UD at the age 14 by an obstetrician gynaecologist. Ultrasound identified that the right uterus was larger than the left. No ureteral or renal anomalies were present. The obstetrician gynaecologist discussed with her that it was optional to have the septum removed but did not discuss the reproductive complications or the potential for dyspareunia. She decided to forego the operation to avoid surgery.
In 2015, she experienced a vaginal bleeding and suspected spontaneous miscarriage at 5 weeks GA, with the loss suspected in known to be smaller left uterus (Figure 1). At baseline she has regular 28 day cycles, menses lasting 7
Figure 1. Ultrasound demonstrating UD following suspected miscarriage.
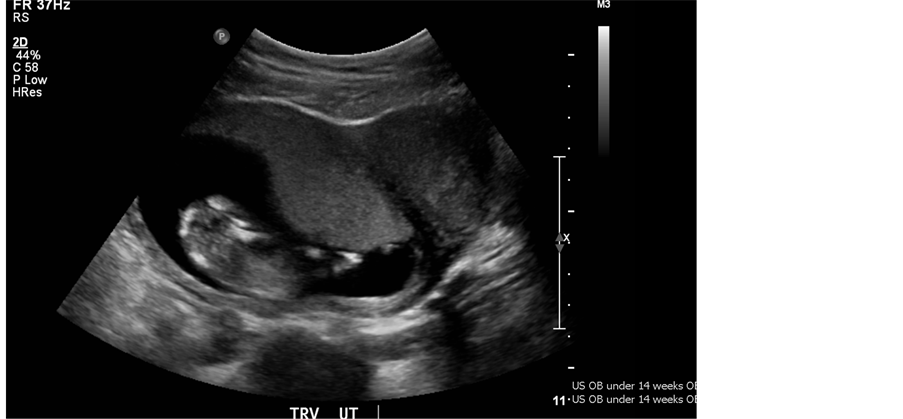
Figure 2. Dating ultrasound at 13 weeks, G2T0P0A1L0 with foetus evident in right uterus of visible UD.
days and flow of moderate volume. She has only ever had PAP smears done for her right cervix (more accessible via speculum exam than the left), has no history of dyspareunia, sexually transmitted infections, pelvic inflammatory disease, nor relevant past medical or surgical history that could impact fertility.
She was referred to the obstetrician gynaecologist at 19 weeks GA following ultrasound confirmation at 13 weeks of a normal foetus in the right uterus with a cervix of 3.7 cm (Figure 2). Antenatal labs, vital signs and genetic screening parameters were all within normal range. Prenatal care was complicated only by persistent spotting from weeks 9 to 11 GA. Anomaly scan at 20 weeks GA was normal. Foetal growth scans at 32 weeks remained promising with healthy vertex foetus, appropriate estimated foetal weight for GA and normal amniotic fluid volumes. Full pelvic assessment at 36 weeks was complicated by inability to identify the vaginal septum since the smaller vaginal wall had collapsed. On repeat pelvic exam at 40 weeks and 6 days GA, a thin, stretchy complete vertical septum was identified. The patient was given the option of elective division of the septum before delivery or to allow division to likely occur naturally during labour. At this point it was deemed safe to offer because she was term. Understanding that labour obstruction is a potential complication of the vaginal septum, she opted for likely division of the septum in labour.
At 41 weeks the patient presented for planned induction of labour. At this time she was contracting irregularly (2 in 10 min) babies head well engaged in the pelvis (0/5 fifths of head palpable above the pubic symphysis), and Bishop’s score > 6 indicating favourable conditions for induction. Longitudinal lie and cephalic presentation were confirmed. Labour was induced with 2 mg Prostaglandin E2 gel applied to right cervix per vagina posterior fornix. She was admitted 5 hours later, 5 cm dilated, 75% effaced, soft, station-1, mid position with moderate show. Spinal anaesthesia was initiated at 7 cm dilation. Amniotomy was performed at 9 cm with clear liquor at 9 hrs post induction. At this time she had a concerning foetal tracing, with prolonged late decelerations (>2 min but less than 10 min) along with the following: variable decelerations, an absence of accelerations, and moderate variability. Subsequent vaginal examination identified the cervix as fully dilated (<9.5 hrs post induction), Station 0 and left occiput anterior presentation. Fatal monitoring continued to be abnormal and second stage of labour was expedited as pushing was commenced with vacuum assisted delivery and mediolateral episiotomy. Spontaneous division of the vaginal septum occurred as the head descended.
She delivered a baby boy with birth weight of 3380 g and had a complete spontaneous tear of the longitudinal vaginal septum relieving the potential obstruction related to the septum. APGAR scores of 21, 35, and 510. A25-minute resuscitation was needed for the baby made a full recovery. The baby recovered with no complications at discharge while mother complains of ongoing dyspareunia possibly related to the naturally torn vaginal septum.
This case highlights the importance of proper counselling, planning, routine follow up and examination needed to optimize the likelihood of having a term delivery in a case of UD. For management of similar cases in a rural setting with a practicing obstetrician where C/S is possible, we propose the following plan as outlined in Table 1. This same plan can be used for planning a term, vaginal delivery following Part B only of the proposed approach outlined in Table 1 below.
Table 1. Delivery and care plan for UD patient in a rural setting with goal of vaginal delivery [2] [31] [32] .
In follow up, the patient volunteered that she had been experiencing some aching and discomfort in her perineal and vulvar area beyond 6 weeks after delivery. She also experienced ongoing secondary post-partum haemorrhaging with persistent vaginal bleeding and passing several large clots up to 6 weeks post partum. This was concluded to most likely represent the expulsion of the left uterine contents that had developed during the gestation. Furthermore, the patient noticed that the vaginal septum that tore during delivery remained attached to one side and was mobile. The septal tissue would protrude 1 cm out the introitus when standing and retract when sitting according to her. This caused her discomfort especially when exercising, sitting on harder surfaces and severe dyspareunia, preventing intercourse with her husband. The remnant septum tissue was subsequently surgically removed by the obstetrician gynaecologist and the patient complained of no further difficulties relating to her UD. The patient has concerns related to potential tearing of the scar tissue in her vaginal canal during subsequent vaginal deliveries.
3. Discussion
Often patients and physicians are both uncertain on how to best manage and approach UD throughout a patient’s life. Literature on management of UD still relies on small retrospective, observational and case studies. As evidenced by this case, standard of care does not exist for this condition and education for obstetricians, family practitioners and patients alike is needed when a diagnosis becomes apparent.
Compared to other MDAs, UD has higher reported rates of term delivery and foetal survival [2] [27] . Despite this, UD patients still exhibit poor reproductive performance with a higher risk of spontaneous abortion, foetal growth retardation, and prematurity (45% decreased chance of carrying to term) [1] [2] [6] [7] [16] [18] [29] [30] [33] . It is speculated that poor expansion of the uterine horn during pregnancy, congenital alternations in vascularisation of the endometrial cavity and abnormal cervical anatomy all contribute to these poorer outcome data [12] .
Cesarean deliveries occur more frequently in patients with Müllerian anomalies because of fetal malpresentation and lack of physician comfort with a trial of normal vaginal delivery [13] [34] . C/S rates for UD are reported at up to 82% [1] [7] [13] . Breech presentation accounts for 43% of C/Sin UD patients with the remaining accounted for by failed trial of labor (concerning fetal heart rate, arrest of labor), previous C/S, preeclampsia, multiple gestation [7] [34] .
Contributing to the C/S rates, are concerns of dystocia caused by the abnormal non-pregnant uterus and those with thick, inelastic vaginal septum’s [1] [2] [7] [13] [15] [35] . Even despite cephalic presentation, providers appear to more often attempt C/S in order to avoid potential dystocia from anatomical obstruction of labour [1] [7] [19] [22] [35] . In a case review, Heinonen identified that it was possible to manually relieve obstruction when they were thin and pliable during delivery [2] [35] . A thick and inelastic vaginal septum, inevitably causes more potential obstructive complications [35] [36] . The antepartum period is not the preferred time of excision of such a septum however due to the potential for triggering pre-term labour [36] . Generally, excision of a thick inelastic septum was preferable prior to pregnancy and also intrapartum to relieve obstruction [35] [36] [37] . In this case, we had a thin, elastic septum running the complete length of the vagina and as with Rezai and de FrançaNeto and colleagues, we allowed the septum to tear naturally and were ready to negotiate any obstructive complications during the intrapartum period. We would like to advocate for the approach by de FrançaNeto for negotiating a vaginal septum in the intrapartum period should it not be removed prior to pregnancy [37] . We support the notion put forth by Rezai and colleagues for removal of the vaginal septum in UD patients only when symptomatic [1] [2] [7] . We do recognize that a thick septum that is obstructive can result in a need for C/S due to failure to progress, but we do not recognize it as an automatic qualification for C/S. Careful monitoring of the peripartum character of the septum is important and anticipatory management is needed.
Overall, UD remains a higher risk pregnancy and a lack of consensus exists in the literature for a consistent approach to antenatal issues and delivery among obstetricians. This case report supports the notion that UD should not be considered an absolute indication for C/S delivery [2] [13] [20] [34] [38] . Consider that there is not even an overt contraindication to trial of labour after C/S (TOLAC) section in UD patients [34] . There is however a lower likelihood of success of TOLAC in UD patients [34] . To our knowledge, there is but one case in the literature describing uterine rupture in a TOLAC [38] .
Hiersch et al. recently identified that overall women with MDAs had higher rates of C/S, however, this increase in rate was not seen in those undergoing a trial of labour [18] . This represents an important consideration and an indication that providers are too cautious with UD cases in traditionally having attempted a trial of labour. We advocate with our colleagues here for the fact that UD is not a contraindication to vaginal delivery in centres with C/S capacity so long as a management strategy is put forward for any complications that may arise [2] [13] [18] [20] [39] . We recommend that if UD is identified pre-preg- nancy, extensive counselling and planning be encouraged for pregnancies and surgical removal of potentially thick, inelastic obstructing vaginal septum be discussed and offered in obstetrical consultation. If UD identified during or known pre-pregnancy, we recommend second trimester obstetrical referral for a detailed evaluation, assessment, and birth plan on a case-by-case basis. Although the C/S rate for UD approaches 82% by some sources, we can take the opportunity to lower this rate by reinforcing the notion that without clear contraindication, even in TOLAC, vaginal delivery is safe to attempt in UD with preparation, foetal monitoring and vigilance throughout the gestation and labour [1] [7] [39] .
4. Conclusion
In conclusion, patients diagnosed with UD require thorough assessment and education for both gynaecological fertility and obstetrical concerns. This case report supports the notion that UD in itself is not an absolute indication for C/S and that a trial of labour is reasonable even in rural centres. More outcome data is needed to improve physician confidence in guiding a trial of labour in patients with UD. The literature base for UD continues to grow and based on current evidence we have provided an approach to management of UD patients in the rural setting.
Acknowledgements
Special thanks to our patient who graciously allowed us to assess her medical records and contribute her case to the literature. Consent was obtained and the Author Confirmation form provided to JOGC.
Conflicts of Interests
The author has no conflict of interests to report.
Cite this paper
Desrochers, J., Boudreau, K. and Adegbenro, A. (2017) Unilateral Singleton Pregnancy with Rural Vaginal Delivery in a Woman with Uterus Didelphys. Open Journal of Obstetrics and Gynecology, 7, 639-648. https://doi.org/10.4236/ojog.2017.76066
References
- 1. Heinonen, P.K. (2000) Clinical Implications of the Didelphic Uterus: Long-Term Follow-Up of 49 Cases. European Journal of Obstetrics and Gynecology, 91, 183-190.
https://doi.org/10.1016/S0301-2115(99)00259-6 - 2. Rezai, S., Bisram, P., Lora Alcantara, I., Upadhyay, R., Lara, C. and Elmadjian, M. (2015) Didelphys Uterus: A Case Report and Review of the Literature. Case Reports in Obstetrics and Gynecology, 2015, Article ID: 865821.
https://doi.org/10.1155/2015/865821 - 3. Jacquinet, A., Millar, D. and Lehman, A. (2016) Etiologies of Uterine Malformations. American Journal of Medical Genetics Part A, 170, 2141-2172.
https://doi.org/10.1002/ajmg.a.37775 - 4. Grimbizis, G.F. (2015) Female Genital Tract Congenital Malformations: Classification, Diagnosis and Management. Springer, London.
https://doi.org/10.1007/978-1-4471-5146-3 - 5. Sanfilippo, J.S. and Peticca, K. (2016) Uterus Didelphys: Diagnosis, Treatment, and Impact on Fertility and Reproduction. In: Pfeifer, S.M., Ed., Congenital Müllerian Anomalies: Diagnosis and Management, Springer International Publishing, Cham, 105-109.
https://doi.org/10.1007/978-3-319-27231-3_9 - 6. Grimbizis, G.F., Camus, M., Tarlatzis, B.C., Bontis, J.N. and Devroey, P. (2001) Clinical Implications of Uterine Malformations and Hysteroscopic Treatment Results. Human Reproduction Update, 7, 161-174.
https://doi.org/10.1093/humupd/7.2.161 - 7. Heinonen, P.K. (1984) Uterus Didelphys: A Report of 26 Cases. European Journal Of Obstetrics Gynecology and Reproductive Biology, 17, 345-350.
https://doi.org/10.1016/0028-2243(84)90113-8 - 8. Chandler, T.M., Machan, L.S., Cooperberg, P.L., Harris, A.C. and Chang, S.D. (2009) Müllerian Duct Anomalies: From Diagnosis to Intervention. The British Journal of Radiology, 82, 1034-1042.
https://doi.org/10.1259/bjr/99354802 - 9. Abo Dewan, K.A.A., Hefeda, M.M. and El Kholy, D.G.E. (2014) Septate or Bicornuate Uterus: Accuracy of Three-Dimensional Trans-Vaginal Ultrasonography and Pelvic Magnetic Resonance Imaging. The Egyptian Journal of Radiology and Nuclear Medicine, 45, 987-995.
- 10. Bermejo, C., Martínez-Ten, P., Recio, M., Ruiz-López, L., Díaz, D. and Illescas, T. (2014) Three-Dimensional Ultrasound and Magnetic Resonance Imaging Assessment of Cervix and Vagina in Women with Uterine Malformations. Ultrasound in Obstetrics & Gynecology, 43, 336-345.
https://doi.org/10.1002/uog.12536 - 11. Rovner, P. and Dovey, S. (2016) Uterine Duplication Anomalies: Distinguishing Among Didelphys, Bicornuate, and Septate Uteri. Topics in Obstetrics & Gynecology, 36, 1-7.
- 12. Giannopoulos, T., Giannopoulos, T. and Croucher, C. (2004) Successful Consecutive Pregnancies in Separate Horns of a Uterus Didelphys. Journal of Obstetrics and Gynaecology, 24, 314.
https://doi.org/10.1080/01443610410001660977 - 13. Rao, S., Anitha, G.S. and Chandralekh,a P. (2016) Pregnancy in Uterus Didelphys Delivered by Caesarean Delivery: Case Report. International Journal of Reproduction, Contraception, Obstetrics and Gynecology, 5, 2434-2437.
https://doi.org/10.18203/2320-1770.ijrcog20162143 - 14. Orazi, C., Lucchetti, M.C., Schingo, P.M.S., Marchetti, P. and Ferro, F. (2007) Herlyn-Werner-Wunderlich Syndrome: Uterus Didelphys, Blind Hemivagina and Ipsilateral Renal Agenesis. Sonographic and MR Findings in 11 Cases. Pediatric Radiology, 37, 657-665.
https://doi.org/10.1007/s00247-007-0497-y - 15. Brown, D.C.D. (1967) Uterus Didelphys and Double Vagina with Delivery of a Normal Infant from Each Uterus. Canadian Medical Association Journal, 96, 675-677.
- 16. Raga, F., Bauset, C., Remohi, J., Bonilla-Musoles, F., Simón, C. and Pellicer, A. (1997) Reproductive Impact of Congenital Müllerian Anomalies. Human Reproduction, 12, 2277-2281.
https://doi.org/10.1093/humrep/12.10.2277 - 17. Zhang, Y., Zhao, Y.-Y. and Qiao, J. (2010) Obstetric Outcome of Women with Uterine Anomalies in China. Chinese Medicine Journal (Engl.), 123, 418-422.
- 18. Hiersch, L., Yeoshoua, E., Miremberg, H., Krissi, H., Aviram, A., Yogev, Y., et al. (2016) The Association between Mullerian Anomalies and Short-Term Pregnancy Outcome. The Journal of Maternal-Fetal & Neonatal Medicine, 29, 2573-2578.
- 19. Browns, O. (1999) Twin Pregnancy in a Uterus Didelphys, with Unilateral Placental Abruption and Onset of Labour. Australian and New Zealand Journal of Obstetrics and Gynaecology, 39, 506-508.
https://doi.org/10.1111/j.1479-828X.1999.tb03146.x - 20. Nohara, M., Nakayama, M., Masamoto, H. and Nakazato, K. (2003) Twin Pregnancy in Each Half of a Uterus Didelphys with a Delivery Interval of 66 Days. BJOG: An International Journal of Obstetrics & Gynaecology, 110, 331-332.
https://doi.org/10.1046/j.1471-0528.2003.01173.x - 21. Heinonen, P.K. (2016) Twin Pregnancy in the Congenital Malformed Uterus. Journal of Obstetrics and Gynaecology, 36, 571-573.
https://doi.org/10.3109/01443615.2015.1103719 - 22. Yang, M.-J., Tseng, J.-Y., Chen, C.-Y. and Li, H.-Y. (2015) Delivery of Double Singleton Pregnancies in a Woman with A Double Uterus, Double Cervix, and Complete Septate Vagina. Journal of the Chinese Medical Association, 78, 746-768.
https://doi.org/10.1016/j.jcma.2015.06.020 - 23. Nhan, V. and Huisjes, H. (1983) Double Uterus with a Pregnancy in Each Half. Obstetrics & Gynecology, 61, 115-117.
- 24. Chan, Y.Y., Jayaprakasan, K., Tan, A., Thornton, J.G., Coomarasamy, A. and Raine-Fenning, N.J. (2011) Reproductive Outcomes in Women with Congenital Uterine Anomalies: A Systematic Review. Ultrasound in Obstetrics & Gynecology, 38, 371-382.
https://doi.org/10.1002/uog.10056 - 25. Maiti, G., Tugnait, P., Anand, A. and Garg, S. (2006) Uterine Didelphys with Pregnancy and Cervical Incompetence. Medical Journal Armed Forces India, 62, 200-201.
https://doi.org/10.1016/S0377-1237(06)80077-8 - 26. Aher, G.S., Gavali, U.G. and Kulkarni, M. (2013) Uterine Didelphys with Cervical Incompetence. International Journal of Medical Research and Health Sciences, 2, 281-283.
https://doi.org/10.5958/j.2319-5886.2.2.012 - 27. Ludmir, J., Samuels, P., Brooks, S. and Mennuti, M.T. (1990) Pregnancy Outcome of Patients with Uncorrected Uterine Anomalies Managed in a High-Risk Obstetric Setting. Obstetrics & Gynecology, 75, 906-910.
- 28. Acién, P., Acién, M. and Sánchez-Ferrer, M.L. (2009) Müllerian Anomalies “without a Classification”: From the Didelphys-Unicollis Uterus to the Bicervical Uterus with or without Septate Vagina. Fertility and Sterility, 91, 2369-2375.
https://doi.org/10.1016/j.fertnstert.2008.01.079 - 29. Acién, P. (1993) Reproductive Performance of Women with Uterine Malformations. Human Reproduction, 8, 122-126.
https://doi.org/10.1093/oxfordjournals.humrep.a137860 - 30. Rackow, B.W. and Arici, A. (2007) Reproductive Performance of Women with Müllerian Anomalies. Current Opinion in Obstetrics and Gynecology, 19, 229-237.
- 31. Lim, K., Butt, K. and Crane, J.M. (2011) SOGC Clinical Practice Guideline. Ultrasonographic Cervical Length Assessment in Predicting Preterm Birth in Singleton Pregnancies. Journal of Obstetrics and Gynaecology Canada, 33, 486-499.
- 32. Perinatal Services BC (2016) Perinatal Tiers of Service Module.
http://www.perinatalservicesbc.ca/health-professionals/professional-resources/system-planning/tiers-of-service - 33. Wallach, E.E. and Jones, H.W. (1981) Reproductive Impairment and the Malformed Uterus. Fertility and Sterility, 36, 137-148.
- 34. Altwerger, G., Pritchard, A.M., Black, J.D. and Sfakianaki, A.K. (2015) Uterine Didelphys and Vaginal Birth After Cesarean Delivery. Obstetrics and Gynecology, 125, 157-159.
- 35. Heinonen, P.K. (1982) Longitudinal Vaginal Septum. European Journal of Obstetrics and Gynecology, 13, 253-258.
https://doi.org/10.1016/0028-2243(82)90106-X - 36. Burkhart, K.P. (1962) Vaginal Soft-Tissue Dystocia. Obstetrics & Gynecology, 20, Article No. 808.
- 37. de Franca Neto, A.H., Nóbrega, B.V., Clementino Filho, J., do ó, T.C. and de Amorim, M.M.R. (2014) Intrapartum Diagnosis and Treatment of Longitudinal Vaginal Septum. Case Reports in Obstetrics and Gynecology, 2014, Article ID: 108973.
- 38. Suthar, S. (2011) Rupture Uterus in Pregnancy with Didelphys Uterus: A Rare Case Report. Journal of South Asian Federation of Obstetrics and Gynaecology, 3, 149-150.
https://doi.org/10.5005/jp-journals-10006-1155 - 39. Magudapathi, C. (2012) Uterus Didelphys with Longitudinal Vaginal Septum: Normal Delivery. Journal of Clinical Case Reports, 2, 194.
Abbreviations List
Caesarean Section: C/S
Gestational Age: GA
Müllerian Duct Anomaly: MDA
Trial of Labour after Caesarean Section: TOLAC
Uterus Didelphys: UD