Advances in Chemical Engineering and Science
Vol. 2 No. 1 (2012) , Article ID: 16721 , 8 pages DOI:10.4236/aces.2012.21021
Aqueous-Phase Sorption Behaviors of Cs+, Co2+, Sr2+ and Cd2+ Ions on Some Composite Ion Exchangers
Hot Laboratories Center, Atomic Energy Authority, Cairo, Egypt
Email: eman_nuclear@yahoo.com
Received November 18, 2011; revised December 25, 2011; accepted January 3, 2012
Keywords: Organic-Inorganic Composite; Polyacrylamide; Tin (IV) Silicate; Tin (IV) Antimonate; Radioactive Waste; Heavy Metals; Sorption Isotherm; Capacity
ABSTRACT
A two new hybrid “organic-inorganic” composite ion exchangers (SAM and FAM), was synthesized by the combination of inorganic ion exchanger tin (IV) silicate and tin (IV) antimonate respectively with organic polymer polyacrylamide (PAm). The sorption isotherms for Cs+, Co2+, Sr2+ and Cd2+ ions on composite ion exchanger were investigated in the range (0.0005 - 0.01 M) at different reaction temperature (30˚C, 45˚C and 60˚C ± 1˚C). The sorption data were subjected to different sorption isotherms and the results verified that Langmuir isotherm is the best model to be applied, and the monolayer sorption capacity were calculated and was found to increase as the reaction temperature increases.
1. Introduction
The long-lived radionuclides in radioactive waste have been considered to be dangerous pollutants, and their migration with groundwater is strongly affected by adsorption on the geologic materials. The presence of radionuclides and toxic metals in wastes is a major environmental concern. Such wastes arise from technologies producing nuclear fuels, and from laboratories working with radioactive materials [1,2]. Various treatment technologies have been developed for the removal of heavy metals from water. The commonly used technologies for removing metal ions from effluents include chemical precipitation, lime coagulation, ion exchange [3,4].
Organic ion-exchangers (ion-exchange resin) possessed high ion-exchange capacity, chemical stability and better regeneration characteristics, while inorganic ionexchangers exhibited higher thermal and radiation stability, rigid structure and undergo negligible swelling during use. To overcome these obstacles, researchers have been motivated to develop organic-inorganic composite cation-exchangers. Composite cation-exchangers possessed striking features as compared to organic as well as inorganic ion-exchange media [5-9]. Also, synthetic organic-inorganic composite cation-exchange materials have received a great deal of attention because of their stability and reproducible analytical and electroanalytical applications [10-17].
In this work, A two new hybrid “organic-inorganic” composite ion exchangers (SAM and FAM) were synthesized, and we studied the sorption isotherm of Cs+, Co2+, Sr2+ as radionuclides and Cd2+ as heavy metal ions on SAM and FAM sorbents as a function of concentration, pH, shaking time and temperature.
2. Materials and Methods
2.1. Chemicals
All reagents were of analytical grade and were used without further purification. Working solutions initating wastewater with a concentration Range from (0.0005 - 0.01 M) metal ions were prepared by appropriate dilutions of the stock solution immediately prior to their use. The pH was adjusted either with diluted HCl or with NaOH solutions.
Radioactive Isotopes and Heavy Metals
The radioactive tracer of 134Cs, 60Co and 90Sr were obtained from Nuclear Resarch Reactor, Inshas, Egypt in the high purity of CsCl, CoCl2 and SrCO3 were prepared by dissolving the respective radioactive salts in 3 M HCl and evaporated to dryness and washed several times with distilled water and finally diluted to the desired volume. The stock solutions were kept in proper stoppered vessels. Also cadmium chloride was prepared as example for heavy metals.
2.2. Synthesis of Composite Ion Exchangers
2.2.1. Preparation of Reagents
Synthesis of Polyacrylamide Polyacrylamide gels were prepared by adding 2% acrylamide in 200 ml DMW. Then, heating the mixture gently at 70˚C with continuous stirring by a magnetic stirrer for 2 h until white gel was obtained.
2.2.2. Synthesis of SAM Sorbent
The hybrid “organic-inorganic” composite cation exchanger poly acrylamide tin (IV) silicate (SAM) was prepared by adding one volume of 1.2 M tin solution prepared in 100 ml aqua regia, then added to 5 volume of sodium silicate. The white precipitates were obtained, when the pH of the mixtures was adjusted 8.4 adding aqueous ammonia with constant stirring. The gels of poly acryl amide were added into the white inorganic precipitate of Sn(IV) silicate mixed thoroughly with constant stirring. These slurries were refluxed for 2 h at a temperature of 70˚C ± 5˚C and were kept for 24 h at room temperature (25˚C ± 2˚C). In this case, the separation process of the precipitate occurred with the use of centrifuge (104 rpm), dried at 50˚C for several days. The obtained product was treated with 0.05 M NaOH solution for 24 h, then washed with bidistilled water, dried at 50˚C ± 1˚C and sieved to obtain different mesh sizes at ambient room temperature.
2.2.3. Synthesis of FAM Sorbent
FAM sorbents were synthesized by adding polyacrlamide gels into the pale white precipitate of inorganic ion exchanger Sn(IV) antimonite which prepared by adding one volume of 1 M tin solution prepared in 100 ml aqua regia, then added to 2 volume of antimony chloride, Mixed thoroughly with constant stirring. These slurries were refluxed for 2 h at a temperature of 70˚C ± 5˚C. The resultant products were left for 24 h at room temperature for digestion. At the final stage, the composite cationexchanger gels were filtered off; washed with demineralize water (DMW) to remove excess acid. The washed gel dried at 40˚C in an oven. The dried product was cracked into small granules and converted into to Na+- form by treating with 0.05 M NaOH for 24 h with occasional shaking intermittently replacing the supernatant liquid with fresh base. The excess base was removed after several washings with DMW and finally dried at 50˚C and sieved to obtain different particles size and kept in desiccators.
2.3. Instrumentations.
2.3.1. Gamma Spectrometer
The radioactive nuclides used in this study 134Cs, 60Co and 90Sr were measured radiometrically liquid phases by γ-ray spectrometry using single channel analyzer (radiometric measurements), model Genie TM2000 CANBERRA, USA.
2.3.2. Atomic Absorption Spectrophotometer
An atomic absorption spectrophotometer model AA- 6701 F-Shimadzu, Kyoto “Japan” was used in the distribution coefficient measurements and also in column chromatography measurements.
2.3.3. Water Thermostat Shaker
Equilibration studies were performed using a water thermostat shaker of type Julabo SW-20C/2, Germany.
2.3.4. pH-Meter
The pH-values of solutions were measured using bench pH meter, model 601A, USA.
2.4. Isotherms Measurements
Sorption isotherm of Cs+, Co2+, Sr2+ and Cd2+ ions were determined over the entire metal ion concentration range from 0.0005 - 0.01 M at constant V/m ratio of 100 ml/g., constant pH of 4 and at different reaction temperature (30˚C, 45˚C and 60˚C). After 24 hours (time sufficient to attain equilibrium) the solution was separated and analyzed for the determination of the residue metal ion concentration. The equilibrium concentration (Ceq) and the amount sorbed (Cads) were calculated using the following relations:
 V/m mmol/g (1)
V/m mmol/g (1)  (2)
(2)
where:
Cads is the amount sorbed of metal ion.
Ceq is theequilibrium concentration.
Co is the initial concentration of metal ion (mmol/g).
V is the solution volume (ml).
M is the weight of the resin (g).
Plot of Ceq against Ceq/Cads or log Ceq against log Cads were performed to obtain the required isotherm.
2.5. Statistical Analysis
Computations were made using Microcal Origin (Version 5.0) software. The goodness of fit was discussed using regression correlation coefficient (r).
3. Results and Discussion
The adsorption isotherm indicates how the adsorption molecules distribute between the liquid phase and the solid phase when the adsorption process reaches an equilibrium state [18]. When an adsorbent comes into contact with a metal ion solution, the concentration of metal ions on the surface of the adsorbent will increase until a dynamic equilibrium is reached; at this point, there is a clearly defined distribution of metal ions between the solid and liquid phases [19]. The analysis of the isotherm data by fitting them to different isotherm models is an important step to find the suitable model that can be used for design purpose [18].
To study the nature of the sorption process of Cs+, Co2+, Sr2+ and Cd2+ ions on SAM and FAM sorbents sorption isotherm were investigated by gradual increase of the sorbate concentration and measuring the amount sorbed at each equilibrium concentration.
The data from sorption were tested and subjected to main commonly used isotherms, namely Langmuir and Freundlich adsorption isotherms. Although the basic assumptions for these models were not fulfilled due to the heterogeneity of the sorbents surface, they were quite successful in predicting the experimental saturation capacities of the sorbents [20-23]. The Langmuir model is probably the best known and most widely applied sorption isotherm. It has produced good agreement with a wide variety of experimental data.
3.1. Freundlich Isotherm Model
Freundlich adsorption isotherm is the relationship between the amounts of metal adsorbed per unit mass of the adsorbent Cads and the concentration of the metal at equilibrium (Ce).
Freundlich equation [24]:
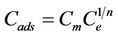 (1)
(1)
The logarithmic form of the equation becomes:
 (2)
(2)
where:
Cads is the amount sorbed onto the sorbent.
Cm is the sorption capacity.
Ce is the equilibrium concentration of the ions in the solution (M).
1/n is the sorption intensity.
By plotting logCads vs. logCe does not fit with this model and the value of R2 is weak by comparison to R2 value of Langmuir isotherm model.
3.2. Langmuir Isotherm Model
The Langmuir isotherm applies to adsorption on completely homogenous surfaces with negligible interaction between adsorbed molecules.
The Langmuir model is developed to represent chemisorption on a set of well-defined localized adsorption sites having same sorption energies independent of surface coverage and no interaction between adsorbed molecules. Maximum sorption capacity (Q) represents monolayer coverage of sorbent with sorbate and b repre sents enthalpy of sorption and should vary with temperature [25,26].
The linear form of the Langmuir isotherm model, adapted for the uptake of species from solution by a solid substrate, can be expressed in the following way [27];
 (1)
(1)
where:
Ce is the steady state concentration of the metal ion in solution in (mg×dm–3).
Cads is the amount of the metal sorbed under steady state conditions in (mg×g–1).
Q is the maximum sorption capacity (mg×g–1).
b is the Langmuir constant “sorption energy” (dm–3×mg–1).
By plotting Ce vs Ce/Cads, straight lines were obtained at different reaction tempertures as shown in Figures 1-8 for Cs+, Co2+, Sr2+ and Cd2+ ions on SAM and FAM sorbent. Figures facilitate the determination of maximum sorption capacity Q and b which calculated from the slope and intercept of the plot Ce vs. Ce/Cads respectively and the values are presented in Tables 1 and 2.

Table 1. Langmuir constants for sorption of Cs+, Co2+, Sr2+ and Cd2+ ions on SAM-sorbent.

Figure 1. Langmuir isotherm for Cs+ ions on SAM sorbent at different reaction temperatures and at pH 4.

Figure 2. Langmuir isotherm for Co2+ ions on SAM sorbent at different reaction temperatures and at pH 4.

Figure 3. Langmuir isotherm for Sr2+ ions on SAM sorbent at different reaction temperatures and at pH 4.

Figure 4. Langmuir isotherm for Cd2+ ions on SAM sorbent at different reaction temperatures and at pH 4.

Figure 5. Langmuir isotherm for Cs+ ions on FAM sorbent at different reaction temperatures and at pH 4.

Figure 6. Langmuir isotherm for Co2+ ions on FAM sorbent at different reaction temperatures and at pH 4.

Figure 7. Langmuir isotherm for Sr2+ ions on FAM sorbent at different reaction temperatures and at pH 4.

Figure 8. Langmuir isotherm for Cd2+ ions on FAM sorbent at different reaction temperatures and at pH 4.

Table 2. Langmuir constants for sorption of Cs+, Co2+, Sr2+ and Cd2+ ions on FAM-sorbent.
The observed increase in the Q sorption capacity and b values with elevated temperature in Tables 1 and 2 indicates the endothermic nature of the adsorption process [28] which in turn suggests the mechanism of Cs+, Co2+, Sr2+ and Cd2+ ions removal by SAM and FAM sorbents is mainly due to chemisorption. The higher r values indicate the applicability of Langmuir isotherm [20].
4. Conclusion
The sorption behavior of Cs+, Co2+, Sr2+ and Cd2+ ions on SAM and FAM sorbent was investigated. It was found that SAM and FAM sorbents is economical and effective sorbents for the above ions. The sorption data was described by the Langmuir isotherm equation and the data in the linearized form of Langmuir equation gave satisfactory correlation coefficients at different concentration ranges. The nature of the sorption process of all the exchangers studied is chemisorption and endothermic.
REFERENCES
- A. K. Kaygun and S. Akyil, “Study of the Behaviour of Thorium Adsorption on PAN/Zeolite Composite Adsorbent,” Journal of Hazardous Materials, Vol. 147, No. 1-2, 2007, pp. 357-362. doi:10.1016/j.jhazmat.2007.01.020
- D. Humeinicu, G. Drochioiu and K. Popa, “Bioaccumulation of Thorium and Uranyl on Saccharomyces Cerevisiae,” Journal of Radioanalytical and Nuclear Chemistry, Vol. 260, No. 2, 2004, pp. 291-293.
- F. Gode and E. Pehlivan, “Adsorption of Cr (III) Ions by Turkish Brown Coals,” Fuel Processing Technology, Vol. 86, No. 8, 2005, pp. 875-884. doi:10.1016/j.fuproc.2004.10.006
- H. A. Qdais and H. Moussa, “Removal of Heavy Metals from Wastewater by Membrane Processes: A Comparative Study,” Desalination, Vol. 164, No. 2, 2004, pp. 105-110. doi:10.1016/S0011-9164(04)00169-9
- A. A. Khan, et al., “Preparation, Physico-Chemical Characterization, Analytical Applications and Electrical Conductivity Measurement Studies of an ‘Organic-Inorganic’ Composite Cation-Exchanger: Polyaniline Sn(IV) Phosphate,” Reactive and Functional Polymers, Vol. 66, 2006, pp. 1649-1663. doi:10.1016/j.reactfunctpolym.2006.06.007
- W. A. Siddiqui, S. A. Khan, et al., “Synthesis, Characterization and Ion-Exchange Properties of a New and Novel ‘Organic-Inorganic’ Hybrid Cation-Exchanger: Poly (Methyl Methacrylate) Zr(IV) Phosphate,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 295, 2007, pp. 193-199. doi:10.1016/j.colsurfa.2006.08.053
- M. M. Abd El-Latif and M. F. El-Kady, Journal of Applied Sciences Research, Vol. 4, 2008, pp. 1-13.
- S. A. Nabi, A. H .Shalla and S. A. Ganai, Separation Purification Technology, Vol. 43, 2008, pp. 164-178.
- Z. Alam and S. A. Nabi, “Synthesis and Characterization of a Thermally Stable Strongly Acidic Cd (II) Ion Selective Composite Cation-Exchanger: Polyaniline Ce(IV) Molybdate,” Desalination, Vol. 250, No. 2, 2010, pp. 515-522. doi:10.1016/j.desal.2008.09.008
- K. G. Varshney, N. Tayal, A. A. Khan and R. Niwas, “Synthesis, Characterization and Analytical Applications of Lead (II) Selective Polyacrylonitrile Thorium (IV) Phosphate: A Novel Fibrous Ion Exchanger,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 181, No. 1-3, 2001, pp. 123-129. doi:10.1016/S0927-7757(00)00750-0
- K. G. Varshney and N. Tayal, Langmuir, Vol. 89, 2001, p. 25.
- K. G. Varshney and P. Gupta, Indian Journal of Chemistry, Vol. 2974, 2003, p. 42.
- A. A. Khan, “Applications of Hg(II) Sensitive Polyaniline Sn(IV) Phosphate Composite Cation-Exchange Material in Determination of Hg2+ from Aqueous Solutions and in Making Ion-Selective Membrane Electrode,” Sensors and Actuators B: Chemical, Vol. 120, 2006, pp. 10-18. doi:10.1016/j.snb.2006.01.033
- A. A. Khan and M. M. Alam, “Synthesis, Characterization and Analytical Applications of a New and Novel “Organic-Inorganic” Composite Material as a Cation Exchanger and Cd (II) Ion-Selective Membrane Electrode: Polyaniline Sn (IV) Tungstoarsenate,” Reactive and Functional Polymers, Vol. 55, No. 3, 2003, pp. 277-290. doi:10.1016/S1381-5148(03)00018-X
- A. A. Khan and M. M. Alam, “New and Novel OrganicInorganic Type Crystalline ‘Polypyrrolel/Polyantimonic Acid’ Composite System: Preparation, Characterization and Analytical Applications as a Cation-Exchange Material and Hg (II) Ion-Selective Membrane Electrode,” Analytica Chimica Acta, Vol. 504, No. 2, 2004, pp. 253- 264. doi:10.1016/j.aca.2003.10.054
- A. A. Khan and M. M. Alam, “Determination and Separation of Pb2+ from Aqueous Solutions Using a Fibrous Type Organic-Inorganic Hybrid Cation-Exchange Material: Polypyrrole Thorium (IV) Phosphate,” Reactive and Functional Polymers, Vol. 63, No. 2, 2005, pp. 119-133. doi:10.1016/j.reactfunctpolym.2005.02.001
- A. A. Khan and M. M. Alam, “Preparation, Characterization and Analytical Applications of a New and Novel Electrically Conducting Fibrous Type Polymeric-Inorganic Composite Material: Polypyrrole Th(IV) Phosphate Used as a Cation-Exchanger and Pb(II) Ion-Selective Membrane Electrode,” Materials Research Bulletin, Vol. 40, No. 2, 2005, pp. 289-305. doi:10.1016/j.materresbull.2004.10.014
- B. H. Hameed, A. L. Ahmad and K. N. A. Latiff, “Adsorption of Basic Dye (Methylene Blue) onto Activated Carbon Prepared from Rattan Sawdust,” Dyes and Pigments, Vol. 75, No. 1, 2007, pp. 143-149. doi:10.1016/j.dyepig.2006.05.039
- M. E. Argun, S. Dursun, C. Ozdemir and M. Karats, “Heavy Metal Adsorption by Modified Oak Sawdust: Thermodynamics and Kinetics,” Journal of Hazardous Materials, Vol. 141, No. 1, 2007, pp. 77-85. doi:10.1016/j.jhazmat.2006.06.095
- C. S. Sundaram and S. Meenakshi, “Fluoride Sorption Using Organic-Inorganic Hybrid Type Ion Exchangers,” Journal of Colloid and Interface Science, Vol. 333, No. 1, 2009, pp. 58-62. doi:10.1016/j.jcis.2009.01.022
- J. S. Allen, J. L. Whitten, M. Murray and O. Duggan, “The Adsorption of Pollutants by Peat, Lignite and Activated Chars,” Journal of Chemical Technology & Biotechnology, Vol. 68, No. 4, 1997, pp. 442-452. doi:10.1002/(SICI)1097-4660(199704)68:4<442::AID-JCTB643>3.0.CO;2-2
- R. Apak, E. T¨utem, M. Hiigiil and J. Hizal, “Heavy Metal Cation Retention by Conventional Sorbents. Red Muds and Fly Ashes,” Journal of Water Resources, Vol. 32, 1998, pp. 430-444.
- M. Yavuz, F. Gode, E. Pehlivan, S. Ozmert and Y. C. Sharma, “An Economic Removal of Cu2+ and Cr3+ on the New Adsorbents: Pumice and Polyacrylonitrile/Pumice Composite,” Chemical Engineering Journal, Vol. 137, No. 3, 2008, pp. 453-461. doi:10.1016/j.cej.2007.04.030
- H. M. F. Freundlich, “Uber Die Adsorption in Lasugen,” Journal of Chemical Physics, Vol. 57, 1906, pp. 385.
- W. Songkasiri, D. T. Reed and B. E. Rittmann, “Biosorption of Neptunium (V) by Pseudomonas Fluorescens,” Radiochimics Acta, Vol. 90, 2002, pp. 785-789. doi:10.1524/ract.2002.90.9-11_2002.785
- A. K. Kaygun and S. Akyil, “Study of the Behaviour of Thorium Adsorption on PAN/Zeolite Composite Adsorbent,” Journal of Hazardous Materials, Vol. 147, No. 1-2, 2007, pp. 357-362. doi:10.1016/j.jhazmat.2007.01.020
- I. Langmuir, “The Adsorption of Gases on Plane Surfaces Of Glass, Mica and Platinum,” Journal of the American Chemical Society, Vol. 40, No. 9, 1918, p. 1361.
- T. S. Anirudhan and P. S. Suchithra, “Humic Acid-Immobilized Polymer/Bentonite Composite as an Adsorbent for the Removal of Copper (II) Ions from Aqueous Solutions and Electroplating Industry Wastewater,” Journal of Industrial and Engineering Chemistry, Vol. 16, No. 1, 2010, pp. 130-139. doi:10.1016/j.jiec.2010.01.006

